This question is about the ionic bond formed between the metal lithium (proton number ) and the non-metal fluorine (proton number ).
Draw a diagram to show what happens when a lithium atom reacts with a fluorine atom.

Important Questions on Atoms Combining
This diagram represents a molecule of a certain gas.
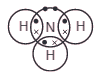
Name the gas, and give is formula.
This diagram represents a molecule of a certain gas.
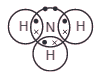
What do the symbols • and x symbols represent?
This diagram represents a molecule of a certain gas.
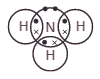
Which type of bonding holds the atoms together?
This diagram represents a molecule of ammonia.
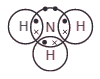
Name another compound with this type of bonding.
Aluminium and nitrogen react to form an ionic compound called aluminium nitride.
Name another non-metal that will form an ionic compound with aluminium, in the same way as nitrogen does.
