EASY
Diploma
IMPORTANT
Earn 100
Two ideal gases and are kept at the same temperature. The gas has molar mass and gas has molar mass . What is the ratio of average speeds of the molecules of gas to that of gas ?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermal Physics
EASY
Diploma
IMPORTANT
EASY
Diploma
IMPORTANT
MEDIUM
Diploma
IMPORTANT
EASY
Diploma
IMPORTANT
MEDIUM
Diploma
IMPORTANT
MEDIUM
Diploma
IMPORTANT
In an experiment, a heater of power is used to warm of a liquid in an uninsulated container. The graph shows the variation of the temperature of the liquid with time.
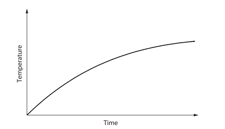
The liquid never reaches its boiling point. Suggest why the temperature of the liquid approaches a constant value.
HARD
Diploma
IMPORTANT
MEDIUM
Diploma
IMPORTANT
The volume of air in a car tyre is about at a temperature of and pressure .
Calculate the number of molecules in the tyre( in no. of Molecules).
