HARD
Earn 100
Using Grignard reagent, suggest synthesis of following alcohol from aldehyde or ketone. Whenever possible, suggest more than one combination.
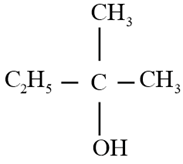

Important Questions on Alcohols, Phenols, and Ethers
MEDIUM
MEDIUM
The aldehydes which will not form Grignard product with one equivalent of Grignard reagents are
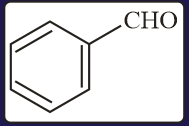
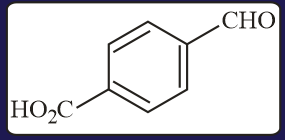
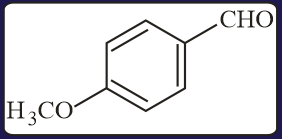
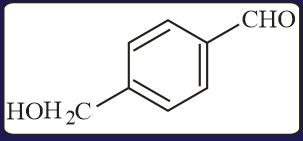
EASY
EASY
HARD
MEDIUM
EASY
MEDIUM
The product of the following reaction sequence is
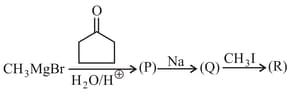
EASY
Propene to propanol
EASY
EASY
MEDIUM
MEDIUM
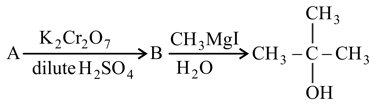 the reactant is:
the reactant is:
EASY
HARD
Consider the following sequence of reactions.
is
EASY
MEDIUM
(i) in tetrahydrofuran solvent and
(ii) alkaline solution is
MEDIUM
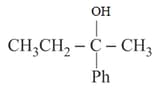
cannot be prepared by:
EASY
Write the structures of the main products in the following reactions:
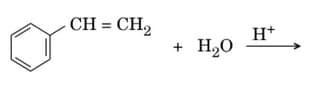
EASY
In the following reaction sequence:
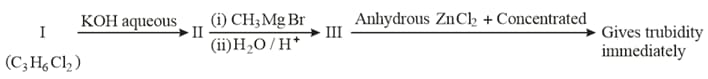
Compound is

