EASY
Earn 100
Using the following information determine the boiling point of a mixture contains 1560 g benzene and 1125 g chlorobenzene, when the external pressure is 1000 torr. Assume the solution is ideal.
Given, Molar mass of benzene = 78
Molar mass of chlorobenzene = 112.5
Temperature
(0oC)
Vapour pressure of benzene
(torr)
Vapour pressure of chlorobenznee (torr)
80
750
120
90
1000
200
100
1350
300
110
1800
400
120
2200
540
(0oC)
(torr)
(a)120oC
(b)110oC
(c)100oC
(d)90oC
50% studentsanswered this correctly
Important Questions on Solutions
EASY
[Assume ionisation of the complex and coordination number of as and that all molecules are present inside the coordination sphere]
MEDIUM
Which of the following relations is correct?
EASY
HARD
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
The vapour pressure vs. temperature curve for a solution solvent system is shown below.
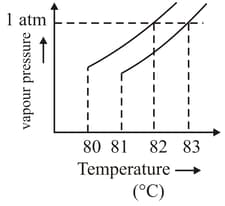
The boiling point of the solvent is _____°C.
MEDIUM
for water is
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
EASY
MEDIUM

