MEDIUM
Earn 100
Vaniya was confused when she came to know about different structures of butane. Deduce the property behind it and give different structures of butane on the basis of this property.
Important Questions on Carbon and its Compounds
HARD
EASY
MEDIUM
HARD
Draw the structures of the isomers for bromobutane.
MEDIUM
MEDIUM
Of the isomeric hexanes, the isomer which can give two mono chlorinated compounds is
EASY
MEDIUM
The following compounds and with the identical molecular formula, but different structures are called;
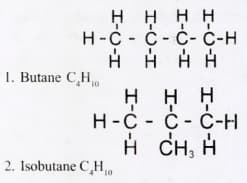
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
HARD
HARD
MEDIUM

