We have a hollow sphere and a solid sphere of equal radii and of the same material. They are heated to raise their temperature by equal amounts. How will the change in their volumes, due to volume expansions, be related? Consider two cases
hollow sphere is filled with air,
there is vacuum inside the hollow sphere.

Important Questions on Thermal Properties of Matter
The figure shows three temperature scales with the freezing and boiling points of water indicated.
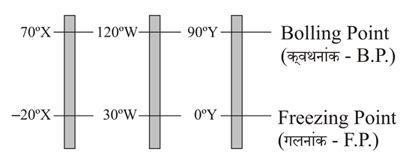
Rank the size of a degree on these scales, greatest first.
Rank the following temperatures, highest first :
A thermally isolated vessel contains of water at . When the air above the water is pumped out, some water freezes and some evaporates at itself. Then the mass of the ice formed if no water is left in the vessel. Latent heat of vaporization of water at and latent heat of fusion of ice.
