What are Diathermanous and Athermanous substances?
Important Questions on Kinetic Theory of Gases and Radiation
The circuit below is used to heat water kept in a bucket.
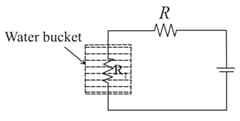
Assuming heat loss only by Newton's law of cooling, the variation in the temperature of the water in the bucket as a function of time is depicted by
In , a body cools from to at room temperature of The temperature of body at the end of next is
A container with of water in it is kept in sunlight, which causes the water to get warmer than the surroundings. The average energy per unit time per unit area received due to the sunlight is and it is absorbed by the water over an effective area of Assuming that the heat loss from the water to the surroundings is governed by Newton's law of cooling, the difference (in ) in the temperature of water and the surroundings after a long time will be_________. (Ignore effect of the container, and take constant for Newton's law of cooling Heat capacity of water )
(Approximate the answer to two decimal place)

