What are the characteristics of chemical equilibrium?
Important Questions on Equilibrium
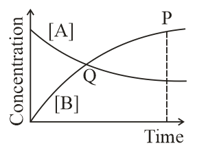
Graph of a reversible process;
is given. Analyse the graph and answer the following question.
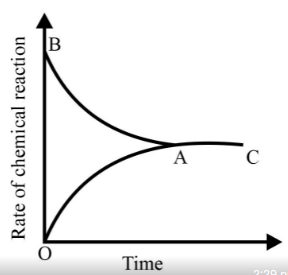
Identify the part of the graph which represents the forward reaction
[ OA, BA, AC]
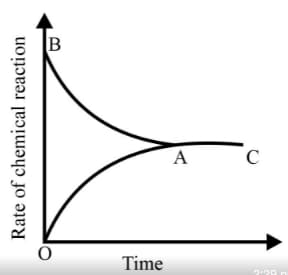
Graph of a reversible process,
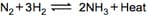
is given. Analyse the graph and answer the following question.
From the given statements, select the correct ones regarding chemical equilibrium.
(i0 The chemical equilibrium is 'static' at the molecular level.
(ii) Both reactants and products co-exist.
(iii) The rates of forward reaction and backward reactions are equal.
(iv) Chemical equilibrium is attained in an open system.
At equilibrium, the mass of each of the reactants and products remains constant.
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
Using the data provided, find the value of equilibrium constant for the following reaction at and atm pressure.
If and are equilibrium constants for the reactions and , respectively, then find out the equilibrium constant for the reaction .
Among the following points indicated on the graph, which one represents equilibrium?
Kp for the reaction
is at certain temperature. What lowest relative humidity of air can be achieved using as drying agent at that temperature ?
Aqueous tension at the given temperature = 16 torr
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
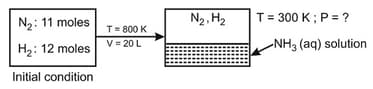
and mixture reacted in vessel at After equilibrium was reached, of was present. of liquid water is pushed in equilibrium mixture and resultant gaseous mixture instantly cooled to Determine the final pressure of gaseous mixture? Neglect vapour pressure of a liquid solution. Assume all dissolved in water no change in volume of liquid no reaction of and at