MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
What are the crystallographic parameter of hexagonal, monoclinic and triclinic unit cell respectively.
Important Questions on Solid State
MEDIUM
JEE Main/Advance
IMPORTANT
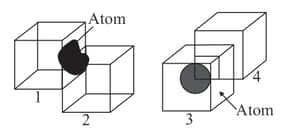
Following diagrams show identical-cubes such that edge of cube? lies exactly in the middle of one of the faces of Cube and Cube has a corner at the body center of the Cube. Find the contributions(in fraction) of the spheres shown to each of the cubes.
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
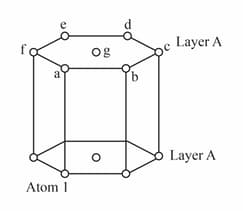
Consider a corner atom of layer of an unit cell showing alternate layer. Find
(i) Find identical atoms ( layer) with respect to the distances from the atom .
(ii) Arrange the distances in ascending order.
MEDIUM
JEE Main/Advance
IMPORTANT
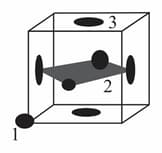
Following figure shows an unit cell with atoms of radius marked (corner ), (face center), (face center). A quadrilateral is also shown by joining the centers of face centered atoms. Find:
(i) The distances between atoms and .
(ii) The shape and dimensions of the quadrilateral.
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
