HARD
Earn 100
What are the formal charges on nitrogen atom 1,2 and 3 respectively ?

(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
Arrange the following compounds in increasing order of bond length: methanol, phenol, -ethoxyphenol
EASY
What do you understand by a lone pair of electrons?
MEDIUM
In ozone molecule, the formal charge on the central oxygen atom is
EASY
Which of the following pair contains lone pair of electrons on the central atom?
MEDIUM
The compound(s) with two lone pairs of electrons on the central atom is (are)
EASY
The number of -bonds and -bonds present in naphthalene are respectively
MEDIUM
Give a reason for the following:
Ionic compounds have a high melting point.
MEDIUM
Two elements and have electronegativities and respectively. The nature of bond between and would be
HARD
The lattice energies of and follow the order
MEDIUM
Which of the following compounds contain(s) no covalent bond(s)?
MEDIUM
The correct order of bond length is:
EASY
The numbers of lone pair and bond pairs in hydrazine are, respectively:
EASY
The intermolecular potential energy for the molecules , , and given below suggests that:
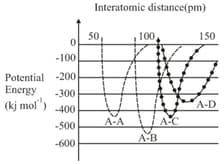
MEDIUM
The compound having longest bond is:
MEDIUM
The compound that has the largest bond angle , is :
MEDIUM
The formal charge on central oxygen atom in ozone is
MEDIUM
Find out the correct order of ionic character in the following molecules
EASY
Total number of lone pair of electrons in ion is:
MEDIUM
Among the following molecules, the one with the largest bond angle at the central atom is
MEDIUM
Maximum bond angle at nitrogen is present in which of the following?

