HARD
Earn 100
What exactly is described by the canonical structure or resonance structures of the hybrid?
Important Questions on Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
Resonance in carbonate ion is
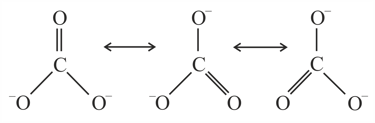
Which of the following is true?
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
[Resonance energy of benzene
Enthalpy of hydrogenation of cyclohexene ]
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
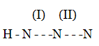
The above compound represents hydrogen azide, the bond orders of bonds and are:
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
Find out the correct order of ionic character in the following molecules
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
HARD
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
The molecule/molecules that has/have delocalised lone pair(s) of electrons is/are
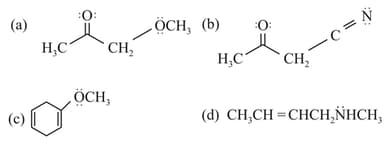
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.

