EASY
Earn 100
What is a heterogeneous equilibrium?
Important Questions on Equilibrium
MEDIUM
MEDIUM
| Initial Concentration (A) | Initial Concentration (B) | Initial rate of formation of C |
|---|---|---|
The rate law for the formation of is
EASY
(assuming ideality)
MEDIUM
MEDIUM
EASY
the equilibrium constant at is . Calculate for the reaction at temperature
HARD
EASY
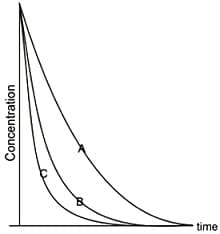
These profiles imply that the decay constants and follow the order
MEDIUM
Using the data provided, find the value of equilibrium constant for the following reaction at and atm pressure.
MEDIUM
EASY
at is If the equilibrium concentration of is the concentration of in is
MEDIUM
EASY
EASY
The value of for the reaction:
is Calculate the value of in .
HARD
The reaction
is in equilibrium in a closed vessel at The partial pressure (in atm) of in the reaction vessel is closest to:
[Given: The change in Gibbs energies of formation at and bar for
Gas constant ]
EASY
HARD
(At )
EASY
At equilibrium, the mass of each of the reactants and products remains constant.
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
EASY
MEDIUM

