EASY
Earn 100
What is critical temperature?
Important Questions on Surface Chemistry
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
Given below are the critical temperatures of some of the gases:
| Gas | Critical temperature |
The gas showing least adsorption on a definite amount of charcoal is
HARD
EASY
EASY
EASY
Match List I with List II
| List I | List II | ||
| A | Physisorption | I | Single Layer Adsorption |
| B | Chemisorption | II | |
| C | III | Chromatography | |
| D | Analytical Application or Adsorption | IV | Heterogeneous catalysis |
Choose the correct answer from the options given below:
EASY
EASY
MEDIUM
EASY
MEDIUM
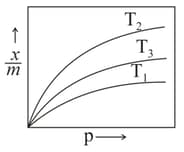
The correct order of the temperatures is:

