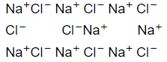EASY
KCET (UG)
IMPORTANT
Earn 100
What is the difference between the number of atoms per unit cell in face-centred cube and the number of atoms per unit cell in body- centred cube?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solid State
EASY
KCET (UG)
IMPORTANT
Potassium crystallises in a lattice. Coordination number of potassium will be:
EASY
KCET (UG)
IMPORTANT
What type of crystal defect is indicated in the diagram below?
EASY
KCET (UG)
IMPORTANT
EASY
KCET (UG)
IMPORTANT
In a crystal the atoms are located at the position of ________.
EASY
KCET (UG)
IMPORTANT
Suppose the mass of a single atom is . metal crystallizes in lattice with unit cell of length . The density of metal in terms of and is______.
EASY
KCET (UG)
IMPORTANT
EASY
KCET (UG)
IMPORTANT
EASY
KCET (UG)
IMPORTANT