What is the effect of intramolecular hydrogen bond on the viscosity of the substance.
Important Questions on Chemical Bonding and Structure
The increasing order of boiling points of the following compounds is :
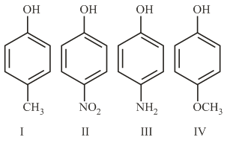
Consider the following molecules and statements related to them:
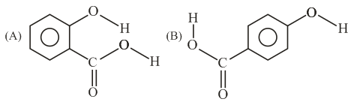
(a) (B) is more likely to be crystaline than (A)
(b) (B) has higher boiling point than (A)
(c) (B) dissolves more readily than (A) in water
Identify the correct option from below:
Given below are two statements:
Statement : -Nitrophenol is steam volatile due to intramolecular hydrogen bonding.
Statement : -Nitrophenol has high melting due to hydrogen bonding.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason .
Assertion : Dipole-dipole interactions are the only non-covalent interactions, resulting in hydrogen bond formation.
Reason : Fluorine is the most electronegative element and hydrogen bonds in are symmetrical.
In the light of the above statements, choose the most appropriate answer from the options given below:

