EASY
NEET
IMPORTANT
Earn 100
What is the hybridisation of positively charged nitrogen atom?
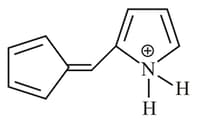

(a)
(b)
(c)
(d)None of these
100% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
In which of the following molecules is the nitrogen atom hybridised?
EASY
NEET
IMPORTANT
What happens to bond angle when reacts with ?
EASY
NEET
IMPORTANT
Which oxide of is paramagnetic?
EASY
NEET
IMPORTANT
In beryllium chloride the hybridization possible in beryllium is:
HARD
NEET
IMPORTANT
Column A describe nature of bonding and Column B the solid having that type of bonding:
| A Nature of bonding) | B (solid) | ||
| I | Van der Waals | P | |
| II | lonic | Q | |
| III | Metallic | R | , diamond |
| IV | Covalent | S | |
Correct matching of A and B is in alternate:
EASY
NEET
IMPORTANT
Hybridization of orbitals of carbon in is necessary to explain which of the following:
EASY
NEET
IMPORTANT
In which of the following, '' atom is hybridized:
MEDIUM
NEET
IMPORTANT
The hybridization of carbon atoms in single bond of is:
