What is the of the following reaction?


Important Questions on Equilibrium
(Given: R and are enthalpy, entropy and Gibbs energy, respectively.)
The gas phase reaction
at has .
The equilibrium constant for this reaction is ___ . (Round off to the Nearest integer)
Use :
antilog
The variation of equilibrium constant with temperature is given below :
The values of at and at (in ) respectively, are close to [use ]
Sucrose Glucose Fructose
If the equilibrium constant is at , the value of at the same temperature will be:
For the equilibrium, which of the following is correct?
The standard free energy change for dissociation of into at and pressure is . The value of is . (Nearest Integer)
[Given : ]
At , ozone is fifty percent dissociated. The standard free energy change at this temperature and pressure is . (Nearest integer)
[Given: and ]
Consider the reaction at . At the time , the temperature of the system was increased to and the system was allowed to reach equilibrium. Throughout this experiment the partial pressure of was maintained at bar. Given below is the plot of the partial pressure of with time. What is the ratio of the standard Gibbs energy of the reaction at to that at ?
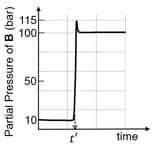
Give your answer by multiplying with 100 and rounding off to nearest integer.
(i)
(ii)
(iii)
(iv)
The reaction with the largest equilibrium constant is

