MEDIUM
JEE Advanced
IMPORTANT
Earn 100
What is the spectrochemical series and its importance?

Important Questions on Coordination Compounds
MEDIUM
JEE Advanced
IMPORTANT
HARD
JEE Advanced
IMPORTANT
MEDIUM
JEE Advanced
IMPORTANT
What would you expect the crystal field stabilisation energy to be and what value of magnetic moment would you expect for the following complexes?
(a)
(b)
(c)
(d)
(e)
HARD
JEE Advanced
IMPORTANT
In the crystal structure of , the is six-coordinate with four at a distance of and two at . Explain the reason for this.
HARD
JEE Advanced
IMPORTANT
Describe and explain the Jahn-Teller effect in the octahedral complexes of and .
HARD
JEE Advanced
IMPORTANT
The complex is diamagnetic, but is paramagnetic and has two unpaired electrons. Explain these observations and deduce the structures of the two complexes.
MEDIUM
JEE Advanced
IMPORTANT
What methods could be used to distinguish between cis and trans isomers of a complex?
HARD
JEE Advanced
IMPORTANT
Name the individual isomers of each of the following:
(a)
(b)
(c)
(d)
(e) 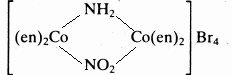
(f)
(g)
