MEDIUM
JEE Main
IMPORTANT
Earn 100
What is the structure of an optical active compound which decolourises bromine water.
(a)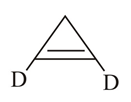
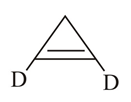
(b)

(c)
(d)
66.67% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
JEE Main
IMPORTANT
An organic compound fused with metallic sodium dissolves in water and the solution is divided into two parts, one part is treated with , boiled and filtered. In the filtrate, addition of does not produce any precipitate. To the other part, addition of sodium nitro prusside produces violet colour. The organic compound contains.
MEDIUM
JEE Main
IMPORTANT
Which of the following is not true for maleic acid and fumaric acid.
MEDIUM
JEE Main
IMPORTANT
The number of optically active stereoisomers possible for butane- -diol.
MEDIUM
JEE Main
IMPORTANT
Among , and , the weakest acid in water is:
MEDIUM
JEE Main
IMPORTANT
The correct order of increasing basicity of the given conjugate bases is:
MEDIUM
JEE Main
IMPORTANT
Which of the following presents the correct order of the acidity in the given compounds?
MEDIUM
JEE Main
IMPORTANT
Base strength is in the order of
and
MEDIUM
JEE Main
IMPORTANT
The correct order of increasing bond length of and is:
