EASY
Earn 100
What type of ligand is? A monodentate, polydentate or an ambidentate.
Important Questions on Coordination Compounds
EASY
Ethylene diaminetetraacetate (EDTA) ion is:
EASY
The donor atom in EDTA are
EASY
Which of the following ligands can act as an ambidentate ligand?
EASY
Explain the term Ligand.
EASY
Which one of the following is an ambidentate ligand?
MEDIUM
What is an ambidentate ligand?
EASY
Which one of the following metal complexes is most stable?
MEDIUM
mol of an octahedral metal complex with formula on reaction with excess of gives of The denticity of Ligand is___________ (Integer answer)
EASY
Which molecule/ion among the following cannot act as a ligand in complex compounds?
EASY
What is the number of nitrogen atoms and - groups respectively present in EDTA?
EASY
What is a bidentate ligand? Give one example.
MEDIUM
The maximum possible denticities of a ligand given below towards a common transition and inner-transition metal ion, respectively, are:
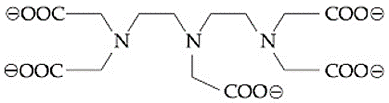
EASY
The total number of coordination sites in ethylenediaminetetraacetate ( ) is ...................
EASY
The denticity of an organic ligand, biuret is :
MEDIUM
The equivalents of ethylene diamine required to replace the neutral ligands from the coordination sphere of the trans-complex of is _______ . (Round off to the Nearest Integer).
EASY
The ligand triethylenetetramine is:
HARD
Compounds with spin-only magnetic moment equivalent to five unpaired electrons are
MEDIUM
Identify monodentate ligand from following
MEDIUM
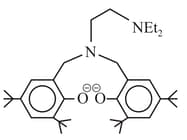
The following ligand is:
MEDIUM
What is the number of donor atoms in dimethylglyoximato ligand?

