MEDIUM
Chemistry
IMPORTANT
Earn 100
What weights of and will be produced by the combustion of of in of oxygen leaving no and .
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Mole Concept
MEDIUM
Chemistry
IMPORTANT
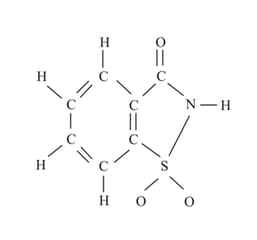
The number of molecules of the sweetener saccharin, which can be prepared
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
From the following reaction sequences:
Calculate the moles of produced by moles of .
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
