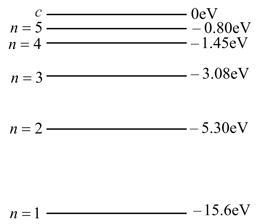EASY
JEE Main/Advance
IMPORTANT
Earn 100
When a hydrogen atom is excited from ground state to first excited state, then,
(a)
its kinetic energy increases by .
(b)
its kinetic energy decreases by .
(c)
its potential energy increases by .
(d)
its angular momentum increases by .
50% studentsanswered this correctly

Important Questions on Atomic Physics
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
The energy levels of a hypothetical one electron atom are shown in the figure