When ammonia gas and hydrogen chloride gas mix together, they react to form a solid called ammonium chloride.
values:
Calculate the molar masses of ammonia, hydrogen chloride and ammonium chloride.

Important Questions on Atoms, Molecules and Stoichiometry
When ammonia gas and hydrogen chloride gas mix together, they react to form a solid called ammonium chloride.
values:
Calculate the volumes of ammonia and hydrogen chloride gases that must react at r.t.p in order to produce of ammonium chloride?
( of gas occupies at r.t.p.).
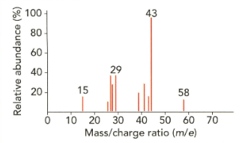
The mass spectrum of an organic compound with the structure is shown.
Identify the fragments with mass/charge ratios of
i. ii. iii. iv.
An organic compound, , has a molecular ion peak, , with a reactive abundance of and an peak with a relative abundance of . Deduce the number of carbon atoms this compound contains.
The mass spectrum of chlorobutane shows an peak beyond the peak. Explain why there is an peak.
The relative height of the peak in organic compound is the same as that of the peak. Explain how this shows that the compound contains one atom of bromine and not one atom of chlorine.