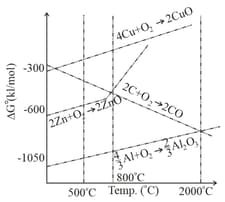
When compared to for the formation of , the for the formation of is
Important Questions on General Principles and Processes of Isolation of Elements (Metallurgy)
The point of intersection and sudden increase in the slope, in the diagram given below, respectively, indicates:
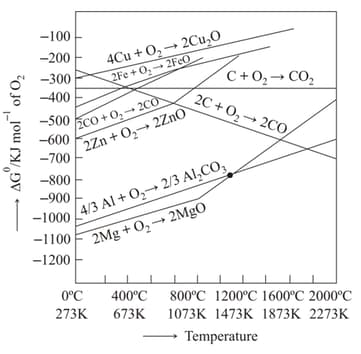
and
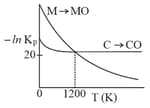
Identify the correct statement:
Given below are two statements.
Statement I: The choice of reducing agents for metals extraction can be made by using the Ellingham diagram, a plot of vs temperature.
Statement II: The value of increases from left to right in the Ellingham diagram.
In the light of the above statements, choose the most appropriate answer from the options given below:
From the Ellingham diagram given below, identify the correct statements
I) Below CO can reduce FeO to Fe
II) Above Coke can reduce FeO to Fe
III CO is the strong reducing agent for the reduction of ZnO to Zn above than coke
IV) Coke reducs ZnO to Zn readily at
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R
Assertion A: In the Ellingham diagram, a sharp change in slope of the line is observed from at
Reason R: There is a large change of entropy associated with the change of state
In the light of the above statements, choose the correct answer from the options given below
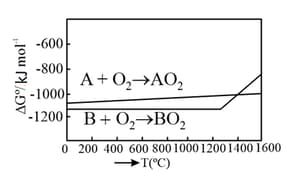
An Ellingham diagram provides information about:
Given below are two statements :
Statement I : According to the Ellingham diagram, any metal oxide with higher is more stable than the one with lower .
Statement II : The metal involved in the formation of oxide placed lower in the Ellingham diagram can reduce the oxide of a metal placed higher in the diagram.
In the light of the above statements, choose the most appropriate answer from the options given below
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason
Assertion A: In an Ellingham diagram, the oxidation of carbon to carbon monoxide shows a negative slope with respect to temperature.
Reason R: CO tends to get decomposed at higher temperature.
In the light of the above statements, choose the correct answer from the options given below
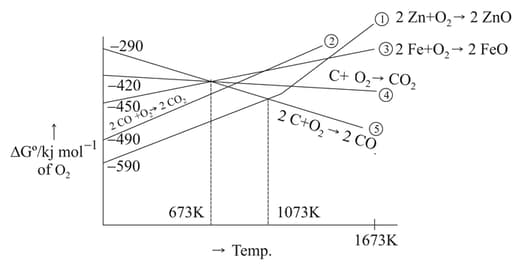
Gibbs energy vs plot for the formation of oxides is given below.
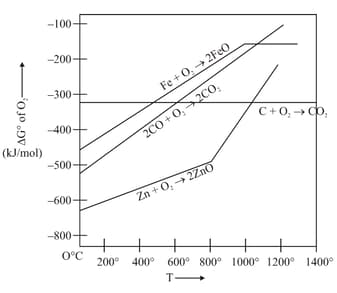
For the given diagram, the correct statement is-
For a reaction
The free energy change is plotted as a function of temperature. The temperature below which the oxide is stable could be inferred from the plot as the point at which :