HARD
JEE Main/Advance
IMPORTANT
Earn 100
When transMethylepoxy pentane treated with aqueous acid:
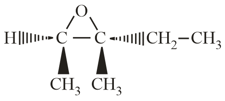 transMethylepoxy pentane
transMethylepoxy pentane
 transMethylepoxy pentane
transMethylepoxy pentane(a)Ring opeing takes place.
(b)The product is chiral.
(c)The product is achiral.
(d)Protonation takes place initially.
100% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
HARD
JEE Main/Advance
IMPORTANT
Which reaction results in the formation of a pair of enantiomers?
HARD
JEE Main/Advance
IMPORTANT
Which of the following reaction is/are not feasible?
HARD
JEE Main/Advance
IMPORTANT
Which of the following reactions takes place by mechanism?
HARD
JEE Main/Advance
IMPORTANT
The relative rates of nucleophilic substitution for the given substrates are as follows:
HARD
JEE Main/Advance
IMPORTANT
Which of the following is/are correct regarding the given reaction?
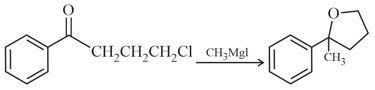
MEDIUM
JEE Main/Advance
IMPORTANT
In the given reaction, the percentage of enantiomer formed is:
MEDIUM
JEE Main/Advance
IMPORTANT
For the reaction: , the rate expression is given as rate What percentage of react by the mechanism when molar.
HARD
JEE Main/Advance
IMPORTANT
In the presence of an anthraquinone derivative as a catalyst, the aqueous solution of sodium dithionite (Fieser's solution) effectively removes oxygen and forms
