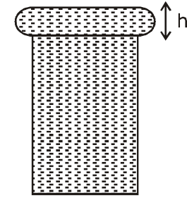
When water is filled carefully in a glass, one can fill it to a height above the rim of the glass due to the surface tension of water. To calculate just before water starts flowing, model the shape of the water above the rim as a disc of thickness having semicircular edges, as shown schematically in the figure. When the pressure of water at the bottom of this disc exceeds what can be withstood due to the surface tension, the water surface breaks near the rim and water starts flowing from there. If the density of water, its surface tension and the acceleration due to gravity are and respectively, the value of is . Find the value of .
(Consider the radius of the disc to be very large compared to the radius of the semicircular edges)

Important Questions on Viscosity and Surface Tension
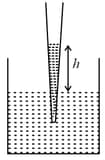
diameter of capillary,
rise of water,
Using and the simplified relation the possible error in surface tension is closest to:
If two glass plates have water between them and are separated by very small distance (see figure), it is very difficult to pull them apart. It is because the water in between forms cylindrical surface on the side that gives rise to lower pressure in the water in comparison to atmosphere. If the radius of the cylindrical surface is R and surface tension of water is T then the pressure in water between the plates is lower by:
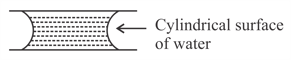
(Surface tension of water

