Which among the following is correct of in normal state?
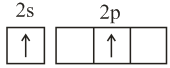
: Against Hund's rule
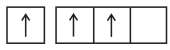
: Against aufbau principle as well as Hund's rule

: Violation of Pauli's exclusion principle and not Hund's rule

: Against aufbau principle
Important Questions on Atomic Structure
Given below are two statements. One is labelled as Assertion and the other is labelled as Reason .
Assertion : Energy of orbital of hydrogen atom is greater than that of orbital of lithium.
Reason : Energies of the orbitals in the same subshell decrease with increase in the atomic number.
In the light of the above statements, choose the correct answer from the options given below.
Which of the following statements are correct?
(A) The electronic configuration of is .
(B) The magnetic quantum number may have a negative value.
(C) In the ground state of an atom, the orbitals are filled in order of their increasing energy order.
(D) The total number of nodes are given by .
Choose the most appropriate answer from the options given below.
(At. No. Z = )

