Which halogen has the largest atomic radius?
Important Questions on Periodic Classification of Elements
The atomic numbers of five elements A, B, C, D and E are and respectively.
(i) Which is the element having the highest electropositivity among these elements? Why?
(ii) Which is the element having the least metallic character among these elements? Why?
(iii) What is your conclusion about the relationship between metallic character and electropositivity of an element?
Which of the following is not a correct statement about the trends when going from left to right across the periods of the Periodic Table?
Study the variation in the atomic radii of second group elements are given below and answer the question given.
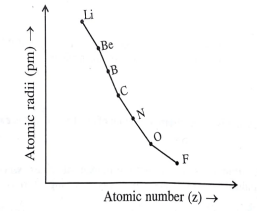
In this second period of elements which element has the smallest atomic size?
Match the left column with the correct answers from the right column.
| Left Column | Right Column |
| Oxide layer protects from attack by water vapour | (a) |
| Group elements of the long periodic table having the least reducing property |
(b) |
| When the metals remain exposed to air, green patches develop on its surface |
(c) |
| Group elements of the long periodic table having the least atomic radius |
(d) |
The position of elements A, B, and C in the Periodic Table are shown below.
| Group 16 | Group 17 |
| - | - |
| - | - |
| - | A |
| B | C |
Will C be larger or smaller in size than B.
The position of elements A, B, and C in the Periodic Table are shown below.
| Group 16 | Group 17 |
| - | - |
| - | - |
| - | A |
| B | C |
State whether A is a metal or non-metal.
Study the variation in the atomic radii of second group elements are given below and answer the question given.
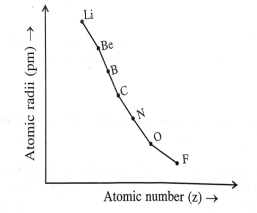
In the second period which element has the highest metallic characteristics?
Give valencies of in the above compounds.
The following table shows the position of six elements A, B, C, D, E, and F.in the modern periodic table:
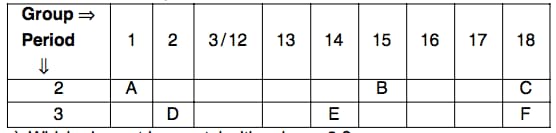
Which element is a non-metal with valency 3?
The following table shows the position of six elements A, B, C, D, E, and F.in the modern periodic table:
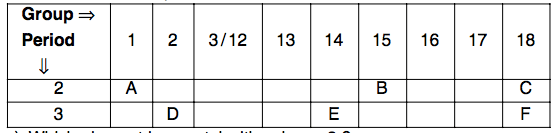
Which element is a metal with valency 2?

