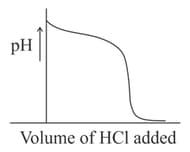
Which is/are correct statements?
(i) When of solution is titrated with solution, the variation of of the solution with the volume of added will be (as shown in the figure):
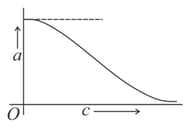
(ii) The variation of the degree of dissociation , with the concentration for a weak electrolyte at a particular temperature is best represented by (as shown in the figure).
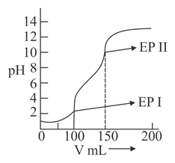
(iii) acetic acid solution is titrated against solution. The difference in between and stages of neutralization of acid will be .



Important Questions on Ionic Equilibrium
A weak acid (or base) is titrated against a strong base (or acid) and the volume V of a strong base (or acid) is plotted against the of the solution (as shown in figure). The weak electrolyte (i.e. acid or base) could be:
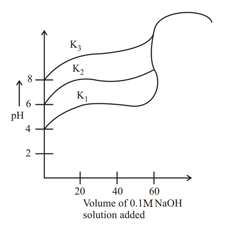
Titration curves for solutions of three weak acids and with ionization constants and respectively are plotted as shown in the figure. Which of the following is/are true?
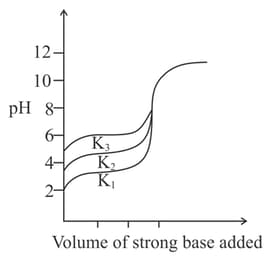
Titration curves for solutions of three weak acids and with ionization constants and , respectively as shown in figure below. The volume of added is in