Which is a double displacement as well as a neutralisation reaction?

Important Questions on Chemical Reactions And Equations
Complete the chemical reaction.
Study the given diagram carefully.
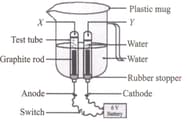
Identify X, Y and the type of reaction occurring.
In this section, each question has two matching lists. Choices for the correct combination of elements from List-I and List-II are given as options (a), (b), (c) and (d) out of which one is correct?
| List-I | List-II |
| (P) | 1. Double displacement |
| (Q) | 2. Combination |
| (R) | 3. Displacement |
| (S) | 4. Decomposition |
In this section, each question has two matching lists. Choices for the correct combination of elements from List-I and List-II are given as options (a), (b), (c) and (d) out of which one is correct?
| List-I | List-II |
| (P) | 1. |
| (Q) | 2. |
| (R) | 3. |
| (S) | 4. |
In this section, each question has two matching lists. Choices for the correct combination of elements from List-I and List-II are given as options (a), (b), (c) and (d) out of which one is correct?
| List-I | List-II |
| (P) | 1. Displacement |
| (Q) | 2. Combination |
| (R) | 3. Decomposition |
| (S) | 4. Neutralisation |
In this section, each question has two matching lists. Choices for the correct combination of elements from List-I and List-II are given as options (a), (b), (c) and (d) out of which one is correct?
| List-I | List-II |
| (P) | 1. Photodecomposition reaction |
| (Q) | 2. Redox reaction |
| (R) | 3. Thermal decomposition reaction |
| (S) | 4. Electrolytic decomposition reaction |
