EASY
Earn 100
Which is the correct graphical representation for ideal gas under isothermal condition?
(a)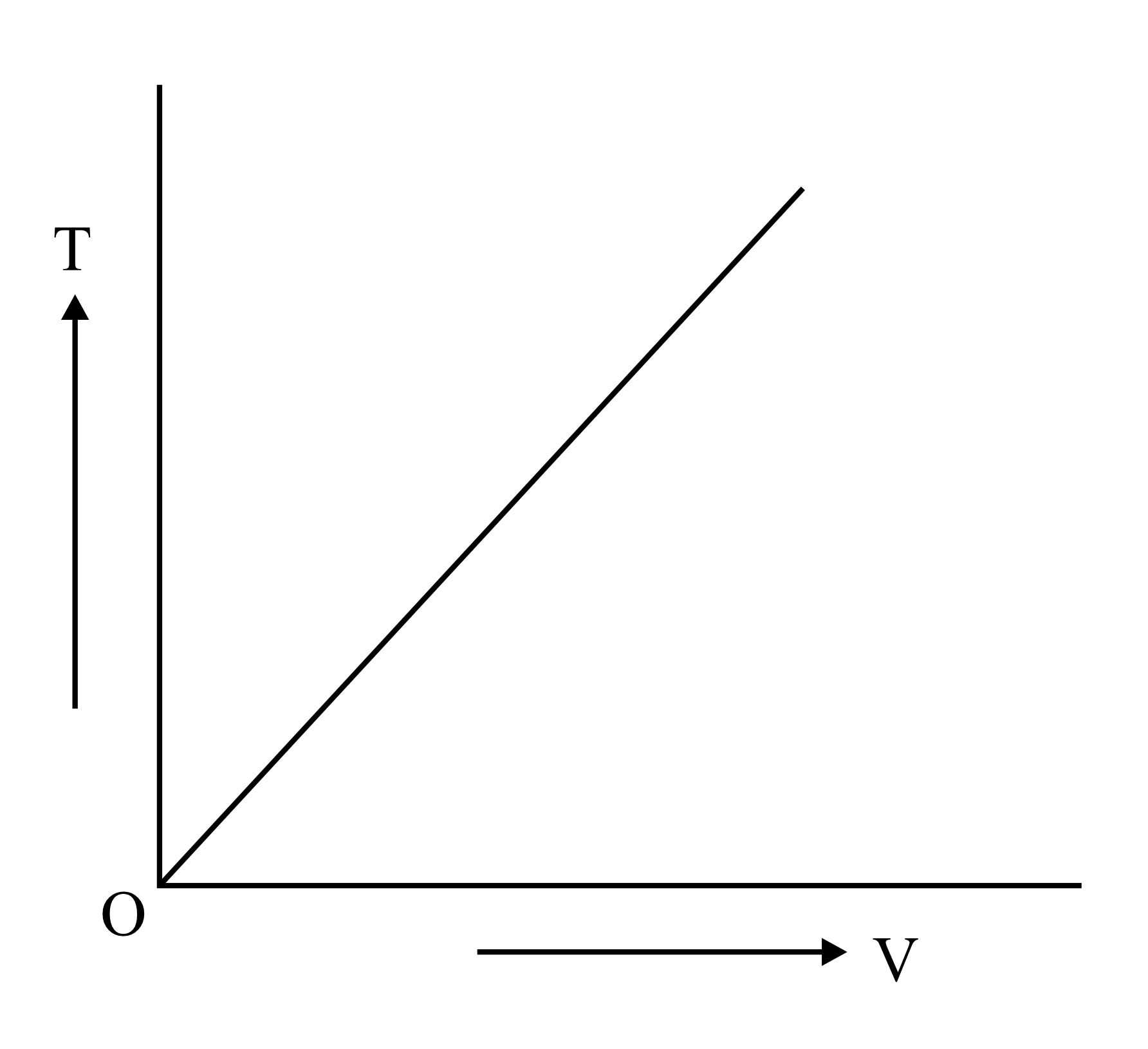

(b)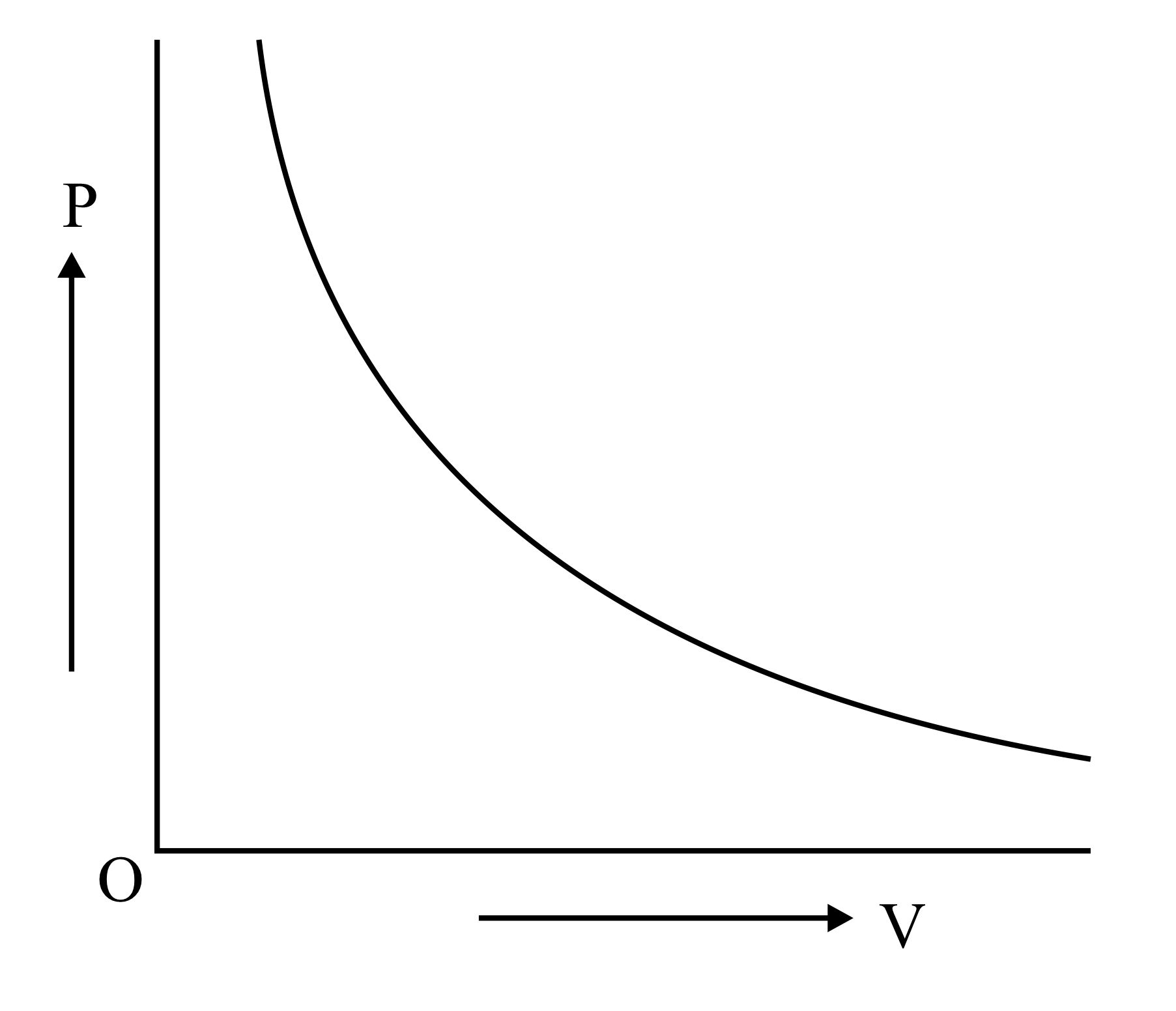

(c)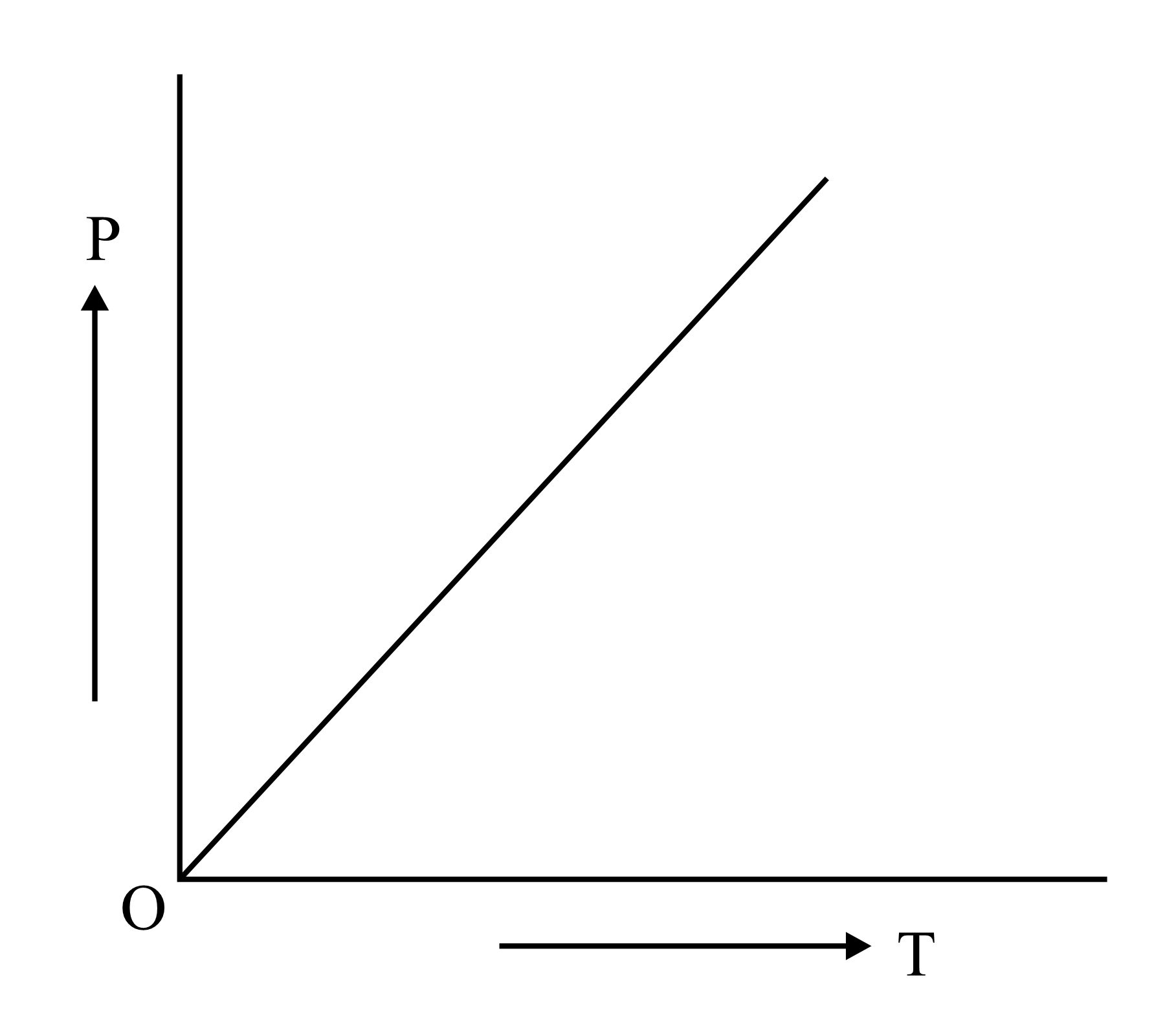

66.67% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
Consider the following thermodynamical variables
(i) Pressure
(ii) Internal Energy
(iii) Volume
(iv) Temperature
Out of these, the intensive variable(s) is (are)
EASY
MEDIUM
A system goes from to via two processes and shown in the figure. If and are the changes in internal energies in the processes I and Il respectively
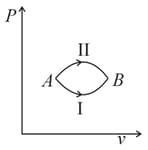
EASY
MEDIUM
MEDIUM
EASY
By what percentage should the pressure of the given mass of gas be increased so to decrease its volume by at a constant temperature?
HARD
Which of the following is isentropic process
MEDIUM
EASY
Consider the following thermodynamic variables:
(i) Pressure
(ii) Internal Energy
(iii) Volume
(iv) Temperature
Out of these, the intensive variable(s) is (are):
MEDIUM
HARD
EASY
Then, equals
EASY
EASY
Which is intensive property?
MEDIUM
EASY
EASY
EASY
MEDIUM

