MEDIUM
Earn 100
Which management principle suggests that each organizational member should receive orders from one superior only?
(a)Unity of Command
(b)Unity of Direction
(c)Order
(d)Discipline
50% studentsanswered this correctly
Important Questions on Coordination Compounds
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
HARD
Four-point charges and are placed at the corners of a square of sideas shown in the figure.
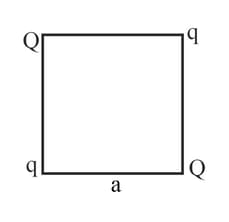
Find the resultant electric force on a charge .
EASY
Explain the term Ligand.
EASY
EASY
EASY
(en ethane-1, 2-diamine)
EASY
EASY
EASY
HARD
Three-point charges and are placed at the vertices of an equilateral triangle ABC of side as shown in the figure. Obtain the expression for the magnitude of the resultant electric force acting on the charge .
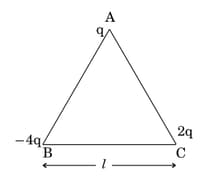
MEDIUM
EASY
MEDIUM
EASY

