EASY
Earn 100
Which of the following are isomers
(a)Methyl alcohol and dimethyl ether
(b)Ethyl alcohol and dimethyl ether
(c)Acetone and acetaldehyde
(d)Propionic acid and propanone
54.29% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
Which of the following pairs of compounds are positional isomers?
MEDIUM
The compound that readily tautomerizes is
MEDIUM
How many isomers are possible for an alkane having molecular formula
MEDIUM
Which of the following carbonyl compounds will exhibit enolization?
(i) 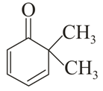
(ii)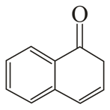
(iii) 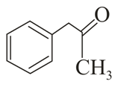
(iv) 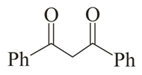
(v) 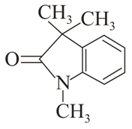
MEDIUM
Which among the given molecules can exhibit tautomerism ?
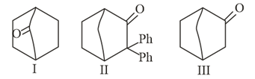
MEDIUM
Tautomerism is not exhibited by:
HARD
Which of the following compounds will show the maximum 'enol' content?
MEDIUM
Assertion A : Enol form of acetone exists in quantity. However, the enol form of acetyl acetone exists in approximately quantity.
Reason R : enol form of acetyl acetone is stabilized by intramolecular hydrogen bonding, which is not possible in enol form of acetone.
Choose the correct statement:
EASY
The maximum number of isomeric ethers with the molecular formula is
EASY
The number of isomers which are ether and having the molecular formula , is:
MEDIUM
The number of isomers of is:
MEDIUM
Given,
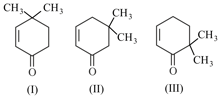
Which of the given compounds can exhibit tautomerism?
EASY
Which of the following is NOT a pair of functional isomers?
MEDIUM
Which one of the following is not an isomer of -methylbut- -yne?
EASY
The following compounds

are
EASY
Which one of the following pairs of isomers is an example of metamerism?
EASY
Compound with molecular formula can show:
MEDIUM
Number of constitutional isomers of alkane with formula is
HARD
The enolic form of ethyl acetoacetate as below has:
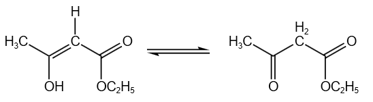
EASY
The correct statement regarding a carbonyl compound with a hydrogen atom on its alpha carbon, is:

