Which of the following elements have same

Important Points to Remember in Chapter -1 - Classification of Elements and Periodicity in Properties from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Brief History of the Development of Periodic Table:
Development of Modern Periodic Table:
(i) Dobereiner's Triads: He arranged similar elements in the groups of three elements called as triads.
(ii) Newland's Law of Octave: He was the first to correlate the chemical properties of the elements with their atomic masses. He observed that the properties of the 8th element were like that of the element.
(iii) Lother Meyer's Classification: He plotted a graph between atomic masses against their respective atomic volumes for several elements. He found the observations:
(a) elements with similar properties occupied similar positions on the curve,
(b) alkali metals having larger atomic volumes occupied the crests,
(c) transitions elements occupied the troughs,
(d) the halogens occupied the ascending portions of the curve before the inert gases and
(e) alkaline earth metals occupied the positions at about the mid points of the descending portions of the curve.
(f) Based on these observations, he concluded that the atomic volumes (a physical property) of the elements are the periodic functions of their atomic masses.
2. Mendeleev's Periodic Table:
Mendeleev's Periodic Law:
The physical and chemical properties of the elements are the periodic functions of their atomic masses.
Merits of Mendeleev's Periodic table:
It has simplified and systematized the study of elements and their compounds.
It has helped in predicting the discovery of new elements based on the blank spaces given in its periodic table.
Demerits in Mendeleev's Periodic Table:
(i) Position of hydrogen is uncertain. It has been placed in and groups.
(ii) No separate positions were given to isotopes.
(iii) Anomalous positions of lanthanides and actinides in periodic table.
(iv) Order of increasing atomic weights is not strictly followed in the arrangement of elements in the periodic table.
(v) Similar elements were placed in different groups.
(iv) It did not explain the cause of periodicity.
3. Modern Periodic Law and the Present Form of the Periodic Table:
Modern Periodic Law (Moseley's Periodic Law):
If the elements are arranged in order of their increasing atomic number, after a regular interval, elements with similar properties are repeated.
Periodicity:
The repetition of the properties of elements after regular intervals when the elements are arranged in the order of increasing atomic number is called periodicity.
The periodic repetition of the properties of the elements is due to the recurrence of similar valence shell electronic configurations after certain regular intervals. The modern periodic table consists of horizontal rows (periods) and vertical column (groups).
Periods:
There are seven periods numbered as and .
(i) Each period consists of a series of elements having same valence shell.
(ii) Each period corresponds to a particular principal quantum number of the valence shell present in it.
(iii) Each period starts with an alkali metal having outermost electronic configuration as .
(iv) Each period ends with a noble gas with outermost electronic configuration except helium having outermost electronic configuration as .
(v) Each period starts with the filling of new energy level.
(vi) The number of elements in each period is twice the number of atomic orbitals available in energy level that is being filled.
Groups:
There are eighteen groups numbered as .
Group consists of a series of elements having similar valence shell electronic configuration.
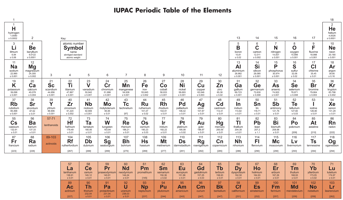
4. Classification of element into four types:
(i) Noble gas:
(a) Element in which the outermost/ultimate shell is completely filled.
(b) General configuration –
(c) Present in group & from to period.
(d) Total element
(ii) Normal or representative element:
(a) Element in which outermost/ ultimate shell is incomplete.
(b) General configuration
(c) Element of subgroup of Mendeleev & from and to group in the long form of periodic table.
(iii) Transition elements:
(a) Elements in which the ultimate as well as penultimate shell are incomplete in ground state as well as in ionic state.
(b) subgroups & group of Mendeleev & from to group of long form of periodic table belongs to a transition element.
(c) According to this concept are not transition element.
(d) General electronic configuration
(iv) Inner transition elements:
(a) Element in which ultimate , penultimate & prepenultimate all the three shells are incomplete.
(b) Elements of (except ) are known as Inner transition element.
5. Classification based on last electron entry:
(i) -block elements: last electron enters in -subshell.
(a) Lies in two group and & period from to .
(b) General formula .
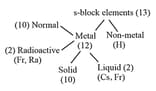
(ii) -block elements: in which last electron enters in -subshell.
(a) Electronic configuration –
(b) to zero group or to group & from period to .
(c) Total elements -
(d) In this block metal, non-metal & metalloid are present.
(iii) -block element in which last electron enters in d-subshell.
(a) lies in between to group.
(b) Period to .
(c) General configuration – .
(iv) -block last electron enters in -subshell.
(a) lies in group & period to period.
(b) General configuration – .
(d) Lanthanoids - found rarely on earth.
(e) Actinoids - All are radioactive.
6. Determination of period, block, and group of an element:
(i) Period number: Maximum principal quantum number in the electronic configuration of an element denotes period number.
(ii) Block number: Can be decided by the shell in which last entered.
Last enters in
-subshell - -block
-subshell - -block
-subshell - -block
-subshell - -block
(iii) Group number:
(a) -block element:
Group Number Number of electrons in ns subshell.
(b) -block element:
Group number Number of electrons in ns + np subshell .
(c) -block element:
Group Number number of electrons in sub shell electrons.
(d) -block element:
Group Number
Q. Predict the period, block & group number of elements having atomic number .
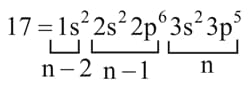
Period ; block ; group number electrons.
7. Nomenclature of Elements in Periodic Table:
(i) Nomenclature:
(a) gave names to elements above atomic No. as follows –
| Nil | Un | bi | tri | quad | pent | hex | sept | oct | Enn |
(b) In all the elements suffix is –
8. Atomic Radii, Ionic Radii and Van der Walls Radii:
(i) Type of Radii:
(a) Covalent radii
(b) Metallic radii
(c) Van der Waal radii
(d) Ionic radii
- Covalent radii:Half of the internuclear distance when two similar atoms bonded together with single bond.
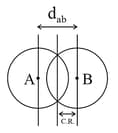
- Metallic radii: Also called as crystal radii. Half of the inter nuclear distance when two adjacent atoms (metal) bonded together with metallic bond.
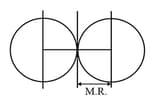
- Van der Waal radii: Half of the internuclear distance between two non-bonded atoms of an inert gas.
Van der Waal radii is almost double of a covalent radius.
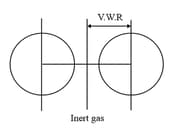
- Ionic radii:
Cationic radii: Cation is always smaller than its parent atom due to either removal of outermost shell or
increased Zeff to the remaining electrons.
Anionic radii: Anion is always more than its parent atom due to increment in inner electrons & decrement in effective nuclear charge (Zeff).
Note: Size of iso-electronic ions: These are such cations or anions which carry the same number of electrons. The size of such ions depends upon the effective nuclear charge. Greater the nuclear charge of an ion, greater will be the force of attraction for same number of electrons. As a result, the size of the ion decreases. For example:
and are isoelectronic ions, among these is largest and is smallest
9. Variation:
(i) Along the period: Radius decreases.
(ii) Down the group: Radius increases.
(iii) In each period maximum measured Atomic radii - Inert gas.
(iv) In each period maximum measured covalent radii - Alkali metal.
(iv) In -block along the period, initially atomic size decreases then becomes constant & then increases due to increment in .
Except:

(v) Size of size of due to lanthanoid contraction.
10. Ionization Enthalpy and its Applications:
lonization Enthalpy:
It is the amount of energy required to remove most loosely held electron from the ground state of an Avogadro number of the isolated atoms, ions, or molecules in the gaseous state. The ion formed by loss of first electron may lose further electrons and thus we may have successive ionization energies for removal of and electrons in the gaseous state.
Ionization is always an endothermic process and ionization energies are therefore always assigned positive values.
10. Factors influencing lonization energy (I.E):
(i) Successive lonization - Generally ionization energy increases for successive ionizations.
(ii) Atomic size - lonization energy decreases as the size of atom increases.
(iii) Value of (atomic number) - Higher the value of , higher is the
(iv) Distance of electron from the nucleus - Smaller the distance of the electron from the nucleus, larger is the ionization energy.
(v) Shielding effect - Higher is the shielding of the electron to be removed, lower is the Shielding effect of the electrons of different orbitals follows the order: .
(vi) Penetration effect - Higher the penetration power of the electron to be removed, higher is the The penetration power of electrons of various orbitals follows the order:
(vii) Nature of shell - lonization energy increases if the electron to be ionized from the species belongs to a half-filled shell or a completely filled shell. The relative stability of these configurations follows the order
(viii) Changes in the quantum shell - During the successive ionization, the electron to be ionized belongs to the lower quantum shell the therefore increases many folds. It is the combined effect of effective nuclear charge, stability of completely filled shells and closer proximity of the lower shell to the positively charged nucleus.
Note:
11. Application of :
(i) Metallic character
(ii) Reactivity of metal
(iii) Basic character
(iv) Number of valence electron: Number of valence electron can be predicted by counting lower value of successive
(v) Number of valence electron Number of lower value.
(vi) Stability of oxidation state:
(a) If then higher oxidation state is stable.
(b) If then lower oxidation state is stable.
12. Electron Gain Enthalpy and its Applications:
Electron affinity and Electron gain Enthalpy
It is defined as the energy released when electron is added to the valence shell of one mole of isolated gaseous atoms or ions and the enthalpy change accompanying the process is defined as the electron gain enthalpy . Electron gain enthalpy provides a measure of the ease with which an atom adds an electron to form anion, . It may be same as of corresponding anion. The first (Electron affinity) of active non-metals is negative. But the addition of a second electron to an already formed anion makes the reaction, endothermic. At the time of formation of oxide or sulphide ion, the effect of 2nd E.A. is so much that the overall for the formation of oxide and sulphide ions is endothermic.
Variation of electron affinity:
(i) Elements which have higher have higher also.
(ii) The values of the second period elements are lower in comparison with the values of third row elements. This is due to increase in the inter electronic repulsions which are more for the smaller elements because of higher electron densities.
(iii) Effective nuclear shielding by the electrons and the necessity of using higher energy orbitals to accept electrons turn the of group elements negative.
(iv) because of very high effective nuclear charge has higher electron affinity.
(v) The electron gain enthalpy of the elements having configuration is positive as electron is to be accommodated into the higher energy orbital.
Note:
Exception: of period of period.
Imp.: Maximum
of of
13. Electronegativity and its Applications:
(i) Electronegativity:
It may be defined as: "The tendency of an atom to attract shared electron pair towards itself in a molecule". The small atoms attract electrons more strongly than larger ones; hence they are more electronegative.
The numerical value of electronegativity depends upon the ionization potential and electron affinity. Higher ionization potential and higher electron affinity both imply higher electronegativity. To measure electronegativity, an arbitrary scale was developed by Linus Pauling which is known as electronegativity scale. On this scale fluorine has maximum electronegativity of and has a value of while inert gas has no value of electronegativity.
The value of electronegativity shows periodic variations as given below:
Generally, in a group electronegativity decreases from top to bottom due to increase in size of atom.
In a period, electronegativity increases from left to right
(ii) Factors:
(a)
(b)
(c) -character
⎯ Max
⎯ Min
⎯ Inert gas has zero value of
Exception: Scale
(iii) According to Mulliken scale: -
Relation between :-
(iv) Application of electronegativity: -
(a) Nature of element
Non-metallic nature
(b) Bond length
(Schomaker & Stevenson law)
(c) Bond strength
(d) Bond energy
(e) Acidic character of hydride in period (left to right)
(f) Nature of oxide
Basic nature
Acidic nature
(g) ionic character
(h) Acidic strength of oxy acid of central atom.
(i) Lewis base strength
(j) Bond polarity
14. Periodic Trends in the Properties of Elements:
(i) Screening effect / Shielding effect :
Repulsive force created by inner electron on a last electron is known as screening effect. In single electron system is absent.
Slater: Rule of calculation of
(a)
(b)
(c)
(d) & all innershell
(ii) Calculation of ( )
[(Number of electrons in shell ) number of electrons in shell number of electrons in shell & all inner shell ]
Variation , along the period & down the group increases.
Order of
(iii) Effective nuclear charge
Net nuclear attraction force exert by nuclei on a valence electron is known as .
For single species
(v) Variation:
(a) Along the period:
Normal element:
increases by
increases by
increases by
(b) -block:
increases by
increases by
increases by
Down the group: Remains constant.
(vi) Lattice Enthalpy:
The lattice enthalpy of an ionic solid is defined as the energy required to completely separate one mole of a solid ionic compound into gaseous constituent ions.
Factors affecting lattice energy of an ionic compound:
(a) Lattice energy where Inter-ionic Distance.
(b) Lattice energy
charge on cation in terms of electronic charge.
charge on anion in terms of electronic charge.
Diagonal relationship:
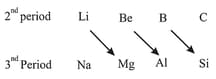
(v) Diagonal relationship arises because of:
(a) On descending a group, the atoms and ions increase in size. On moving from left to right in the periodic table, the size decreases. Thus, on moving diagonally, the size remains nearly the same.
(b) It is sometimes suggested that the diagonal relationship arises because of diagonal similarity in electronegativity values.
Acidic and basic characters of oxides and hydroxides:
In a group, basic nature of oxides increases, or acidic nature decreases. Oxides of the metals are generally basic, and oxides of the non-metals are acidic. The oxides of the metalloids are generally amphoteric in nature. The oxides of and are amphoteric.
In a period, the nature of the oxides varies from basic to acidic.
| Strongly basic | Basic | amphoteric | Weakly acidic | Acidic | Acidic | Strongly acidic |


15. Valency and Oxidation States:
Valency: Combining capacity of an atom:
Two concepts:
(i) Old concept
w.rt. valency of an element in a period initially increases from to & then decrease to .
w.r.t. along the period increases from 1 to .
(ii) New concept (electronic concept):
The number of electrons required to gain or donate to achieve the nearest inert gas configuration.
For example: one electron required to donate i.e., valency
One electron required to gain i.e., valency
16. Valency in a Group remains the Same:
Inert pair effect: The outer shell 's' electrons penetrate to electrons and thus become closer to the nucleus and are more effectively pulled towards the nucleus. This results in less availability of electron pair for bonding of the electron pair becomes inert. The inert pair effect begins after and increases with increases value of .
(i) Dominance of lower oxidation state on moving down the group in boron, carbon & nitrogen family may also be explained by the inert pair effect.
(ii) The inert pair effect is not the explanation of monovalency, bivalency and trivalency in group . It merely describes what happens, i.e., two electrons do not participate in bonding. The reason that they do not take part in bonding in energy.
Order of stability of oxidation state according to inert pair effect

