HARD
Earn 100
Which of the following facts is not true?
(a)If is negative, will be reduced to by the metal
(b)If is positive, will be reduced to by
(c)In a cell electrode is attached to hydrogen half cell. To produce spontaneous reaction, metal will act as negative electrode.
(d)Compounds of active metals are reducible by where as those of noble metals are not reducible.
37.5% studentsanswered this correctly
Important Questions on Electrochemistry
MEDIUM
HARD
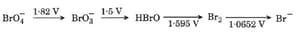
Then, what is the species undergoing disproportionation?
MEDIUM
MEDIUM
What will be the oxidation potential for the following hydrogen half cell at bar pressure and temperature?
EASY
Among the following, the strongest reducing agent is:
EASY
EASY
EASY
MEDIUM
Based on these data, which of the following statements is correct?
HARD
The for will be :
MEDIUM
In which metal container, the aqueous solution of can be stored?
MEDIUM
MEDIUM
EASY
Given the standard half-cell potentials of the following as
Then the standard e.m.f. of the cell with the reaction is
MEDIUM
Consider the cell at
The fraction of total iron present as ion at the cell potential of is . The value of is ______. (Nearest integer)
MEDIUM
(A) Sublimation enthalpy
(B) Ionisation enthalpy
(C) Hydration enthalpy
(D) Electron gain enthalpy
The total number of above properties that affect the reduction potential is (Integer answer)
EASY
Consider the following electrodes
electrode potentials of the above electrodes in volts are in the order
MEDIUM
Calculate the standard cell potential (in V) of the cell in which the following reaction takes place:
Given that
MEDIUM
HARD
The standard reduction potential for is Calculate the reduction potential at for the above couple.

