MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following graphs represents vs for an ideal gas at constant and ?
(a)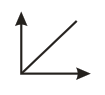

(b)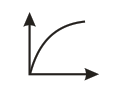

(c)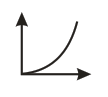

(d)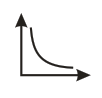

66.67% studentsanswered this correctly

Important Questions on States of Matter
MEDIUM
JEE Main
IMPORTANT
For a fixed mass of a gas at constant pressure, which of the following statements is/are not correct?
MEDIUM
JEE Main
IMPORTANT
Boyle's law cannot be expressed as
HARD
JEE Main
IMPORTANT
Which of the following plots is/are correct?
MEDIUM
JEE Main
IMPORTANT
Which of the following curve does not represent the Boyle's law?
MEDIUM
JEE Main
IMPORTANT
Which of the following plots is/are correct?
MEDIUM
JEE Main
IMPORTANT
What should be the percentage increase in pressure for a decrease in volume of a gas at constant temperature?
HARD
JEE Main
IMPORTANT
The pressure exerted by of an ideal gas at temperature in a vessel of volume litre is one . When the temperature is increased by degree at the same volume, the pressure increases by . Calculate the temperature and volume . (Molar mass of the gas).
HARD
JEE Main
IMPORTANT
of a boron-hydrogen compound created a pressure of in a bulb of at . Analysis showed it to be boron. Calculate its molecular formula.
