EASY
12th West Bengal Board
IMPORTANT
Earn 100
Which of the following graphs represents exothermic reaction?
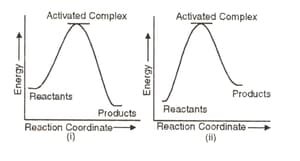
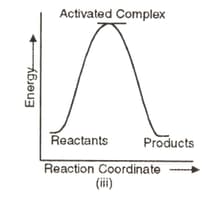


(a)(i) only
(b)(ii) only
(c)(iii) only
(d)(i) and (ii)
33.33% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
12th West Bengal Board
IMPORTANT
Rate law for the reaction is found to be
Rate
Concentration of reactant ' ' is doubled. Keeping the concentration of '' constant, the value of rate constant will be :
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
MEDIUM
12th West Bengal Board
IMPORTANT
Compounds '' and '' react according to the following chemical equation
Concentration of either '' or '' were changed keeping the concentrations of one of the reactants constant and rates were measured as a function of initial concentration. Following results were obtained. Choose the correct option for the rate equations for this reaction.
| Experi- ment | Initial concentration of |
Initial concentration of | Initial rate of formation of |
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
