Which of the following has the intramolecular hydrogen bond?
Important Questions on States of Matter
In which of the following solid substance dispersion forces exist?
[ is the distance between the polar molecules]
Three types of potential energy due to attractive interaction between two species and are represented by the curves and in the figure below.
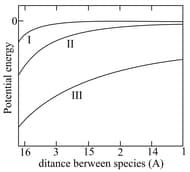
Consider the dominating interaction between non-rotating species for , and . The correct assignment of the ... interactions
to the types , and is:
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |
Increasing order of boiling points in the following compounds is:
Intermolecular forces are forces of attraction and repulsion between interacting particles that will include :
A. dipole-dipole forces.
B. dipole-induced dipole forces.
C. hydrogen bonding.
D. covalent bonding.
E. dispersion forces.
Choose the most appropriate answer from the options given below:

