HARD
Earn 100
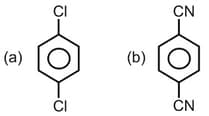
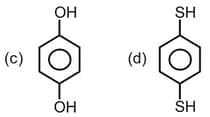
Which of the following hydrocarbon has the lowest dipole moment ?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding
EASY
MEDIUM
EASY
EASY
EASY
EASY
HARD
EASY
HARD
EASY
HARD
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
Which compound among the following will have a permanent dipole moment?
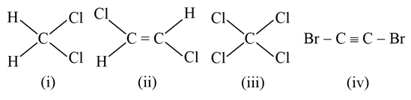
MEDIUM
EASY
HARD