EASY
Earn 100
Which of the following is NOT the function of catalyst?
(a)To increase the rate constant
(b)To influence the forward and backward reactions to the same extent
(c)To reduce the time required for reaching the equilibrium state.
(d)To alter the Gibbs energy change of the reaction
(e)All of these
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
(Assume Activation energy and pre-exponential factor are independent of temperature; )
MEDIUM
MEDIUM
EASY
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)
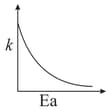
(II)
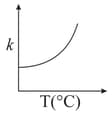
MEDIUM
The Arrhenius plots of two reactions, I and II are shown graphically-
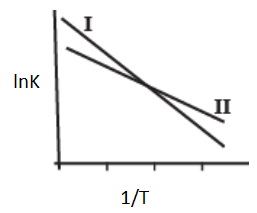
The graph suggests that-
EASY
HARD
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
EASY
EASY
MEDIUM
EASY
MEDIUM
Identify the incorrect statement.
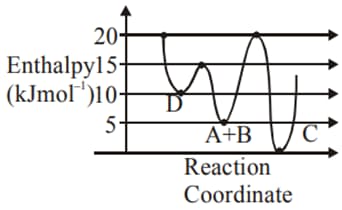
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
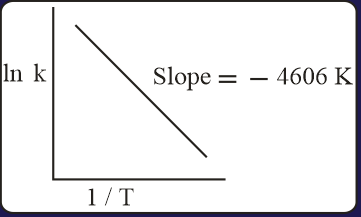
MEDIUM
MEDIUM
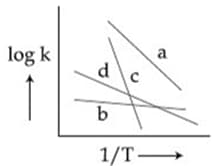
MEDIUM
MEDIUM
MEDIUM
[Gas constant, ]
EASY

