EASY
Earn 100
Which of the following is a intensive property?
(a)Temperature
(b)Viscosity
(c)Surface tension
(d)All of these
50% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
EASY
EASY
MEDIUM
EASY
HARD
EASY
MEDIUM
The total number of intensive properties from the following is _____.
Volume, Molar heat capacity, molarity, ,Gibbs free energy change, Molar mass, Mole
EASY
MEDIUM
MEDIUM
EASY
EASY
Among the following the number of state variable is
Internal energy
Volume
Heat
Enthalpy
EASY
Reversible process
EASY
Observe the following properties:
Volume, enthalpy, density, temperature, heat capacity, pressure, internal energy.
The number of extensive properties in the above list is
MEDIUM
HARD
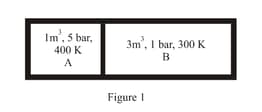
EASY
EASY
EASY
i)
ii)
iii)
iv)

