EASY
10th CBSE
IMPORTANT
Earn 100
Which of the following is acidic in nature?
(a)Lime juice
(b)Human blood
(c)Lime water
(d)Antacid
59.38% studentsanswered this correctly

Important Questions on Acids, Bases and Salts
EASY
10th CBSE
IMPORTANT
In an attempt to demonstrate conductivity through an electrolyte, the following apparatus (figure) was set up.
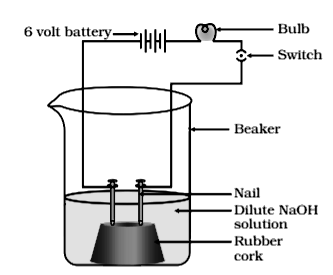
Which among the following statement(s) is/are correct?
(i) Bulb will not glow because the electrolyte is not acidic.
(ii) Bulb will glow because is strong base and furnishes ions for conduction.
(iii) Bulb will not glow because the circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
EASY
10th CBSE
IMPORTANT
Which of the following is used for dissolution of gold?
EASY
10th CBSE
IMPORTANT
Which of the following is not a mineral acid?
EASY
10th CBSE
IMPORTANT
Which among the following is not a base?
EASY
10th CBSE
IMPORTANT
Which of the following statements is not correct?
MEDIUM
10th CBSE
IMPORTANT
Match the chemical substances given in Column I with their appropriate application given in Column II.
| Column I | Column II |
| A. Bleaching powder | 1. Preparation of glass |
| B. Baking soda | 2. Production of and |
| C. Washing soda | 3. Decolourisation |
| D. Sodium chloride | 4. Antacid |
MEDIUM
10th CBSE
IMPORTANT
Equal volume of hydrochloric acid and sodium hydroxide solution of same concentration are mixed and the of the resulting solution is checked with a paper. What would be the colour obtained?
EASY
10th CBSE
IMPORTANT
Which of the following is/are true when is passed through water?
(i) It does not ionise in the solution as it is a covalent compound.
(ii) It ionises in the solution.
(iii) It gives both hydrogen and hydroxyl ion in the solution.
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule.
