EASY
Earn 100
Which of the following is correct regarding diad axis of symmetry?
(a)Diad axis of symmetry is passing through the centre of the two opposite edges.
(b)In the cubical lattice there are three diad axis of symmetry.
(c)Diagonal plane of symmetry is same to the diad axis of symmetry.
(d)All of the above are correct.
4.35% studentsanswered this correctly
Important Questions on Solid State
EASY
A unit cell of calcium fluoride has four calcium ions. The number of fluoride ions in the unit cell is
EASY
Which of the following is NOT true about the amorphous solids?
EASY
In calcium fluoride, having the fluorite structure, the coordination numbers for calcium ion and fluoride ion are
MEDIUM
The contribution to the total current in a semiconductor, due to electrons and holes are and respectively. The drift velocity of electrons is times that of holes at this temperature. Then the ratio between electron concentration and hole concentration is
HARD
Define Anisotropy. Distinguish between crystalline solids and amorphous solids.
EASY
The barrier potential of a p-n junction depends on:
type of semi-conductor material
amount of doping
temperature
Which one of the following is correct?
HARD
Consider an ionic solid , with structure. Construct a new structure , whose unit cell is constructed from the unit cell of , following the sequential instructions given below. Neglect the charge balance.
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
EASY
In the following circuit diagram, the current through the battery is
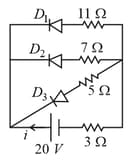
MEDIUM
The material that soften on heating to finally flow like a liquid is _____.
MEDIUM
Give two differences between Crystalline solids and Amorphous solids.
HARD
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
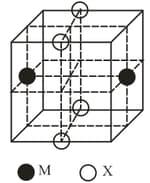
MEDIUM
Given below are two statements. One is labelled as
Assertion and the other is labelled as Reason .
Assertion Sharp glass edge becomes smooth on heating it up to its melting point.
Reason The viscosity of glass decreases on melting.
Choose the most appropriate answer from the options given below.
EASY
An intrinsic semiconductor is converted into -type extrinsic semiconductor by doping it with:-
MEDIUM
The radius of is and that of is . The volume of the unit cell of expressed in is:
EASY
Among the following, which have highest melting point?
EASY
Which among the following is NOT a polar molecular solid ?
EASY
Silicon doped with gallium forms
HARD
Which of the following statement is correct?
EASY
Which of the following statements is true with regards to crystalline solids?

