Which of the following is oxidised by oxygen in acidic medium?
Important Questions on Redox Reactions and Electrochemistry
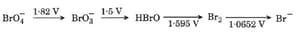
Then, what is the species undergoing disproportionation?
In the following passage there are blanks, each of which has been numbered. There are questions given below the passage and for each question, four words are suggested, one of which fits the blank appropriately. Find out the appropriate word in each case.
Whenever I go into a bank, I feel scared. Everybody and everything that I see there (1) ____ me. As for the manager the sight (2) ____ him simply terrifies me and (3) ____ me want to runaway (4) _____ I can. As soon as I (5) ____ the door of the bank I lose my head (6) ____ when I try to do any (7) ____ there, I behave like an idiot. I cannot explain (8) ____ for this but that is how it (9) ____ has been that is how it is (10) ____.
Select the most appropriate option for blank No. 10.
Given the standard half-cell potentials of the following as
Then the standard e.m.f. of the cell with the reaction is
To a solution containing of and of , the metal rods and are inserted at and connected by a conducting wire. This resulted in dissolution of . The correct combination(s) of and , respectively, is(are)
(Given: Gas constant, , Faraday constant, )
Calculate the standard cell potential (in V) of the cell in which the following reaction takes place:
Given that
In which metal container, the aqueous solution of can be stored?
Among the following, the strongest reducing agent is:
Based on these data, which of the following statements is correct?
What will be the oxidation potential for the following hydrogen half cell at bar pressure and temperature?
The for will be :
The standard reduction potential for is Calculate the reduction potential at for the above couple.

