Which of the following is required for the formation of an ionic bond?
Important Questions on Chemical Bonding and Molecular Structure
For the molecules and what is the correct arrangement of bonds in order of increasing ionic character?
Assuming is an ionic compound and are the ionic radii of and respectively. The lattice energy of the compound is proportional to:
Match the following species with the correct number of electrons present in them:
| Species | Number of Electrons |
Thus process of formation of in gas phase is unfavourable though is isoelectronic with neon. It is due to the fact that,
The electronic configuration of element X is and the electronic configuration of element Y is . Then the type of bond formed between these two elements is
Give a reason for the following:
Ionic compounds have a high melting point.
Find out the correct order of ionic character in the following molecules
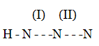
The above compound represents hydrogen azide, the bond orders of bonds and are:
What should be the correct order of lattice energy values of the following alkali halides?
and
Two elements and have electronegativities and respectively. The nature of bond between and would be

