Which of the following is the correct statement of second law of thermodynamics?
Important Questions on Thermodynamics
(Specific heat of water liquid and water vapour are and ; heat of liquid fusion and vaporization of water are and , respectively). ( )
Which of the following show an increase in entropy?
I. Boiling of water
II. Melting of ice
III. Freezing of water
IV. Formation of hydrogen gas from water
During which of the following processes, does entropy decrease ?
(A) Freezing of water to ice at
(B) Freezing of water to ice at
(C)
(D) Adsorption of and lead surface
(E) Dissolution of in water
For the following reaction
and . What is the value of at ?
A container is divided into two compartments by a removable partition as shown below:
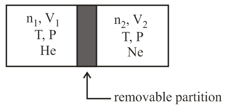
In the first compartment, moles of ideal gas He is present in a volume In the second compartment, moles of ideal gas is present in a volume The temperature and pressure in both the compartments are and respectively. Assuming is the gas constant, the total change in entropy upon removing the partition when the gases mix irreversibly is:

