EASY
Earn 100
Which of the following metals react with dilute hydrochloric acid?
(a)Magnesium
(b)Aluminium
(c)Iron
(d)Copper
50% studentsanswered this correctly
Important Questions on Metals and Non-Metals
EASY
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
Write a chemical equation for the reaction taking place in the flask. Write name and one property of the gas evolved.
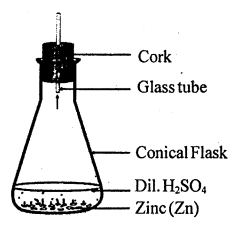
MEDIUM
What are amphoteric oxides? Give an example.
EASY
MEDIUM
MEDIUM
Answer the following:
Why are highly reactive metals such as Aluminium is used as reducing agents?
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
Why is sodium kept immersed in kerosene oil?
EASY
Attempt the following:
The metal sodium is kept immersed in Kerosene oil in the school laboratory. Why it is not kept the open?
EASY

