MEDIUM
Earn 100
Which of the following methods will form cyclopentyl ethyl ether
(a)
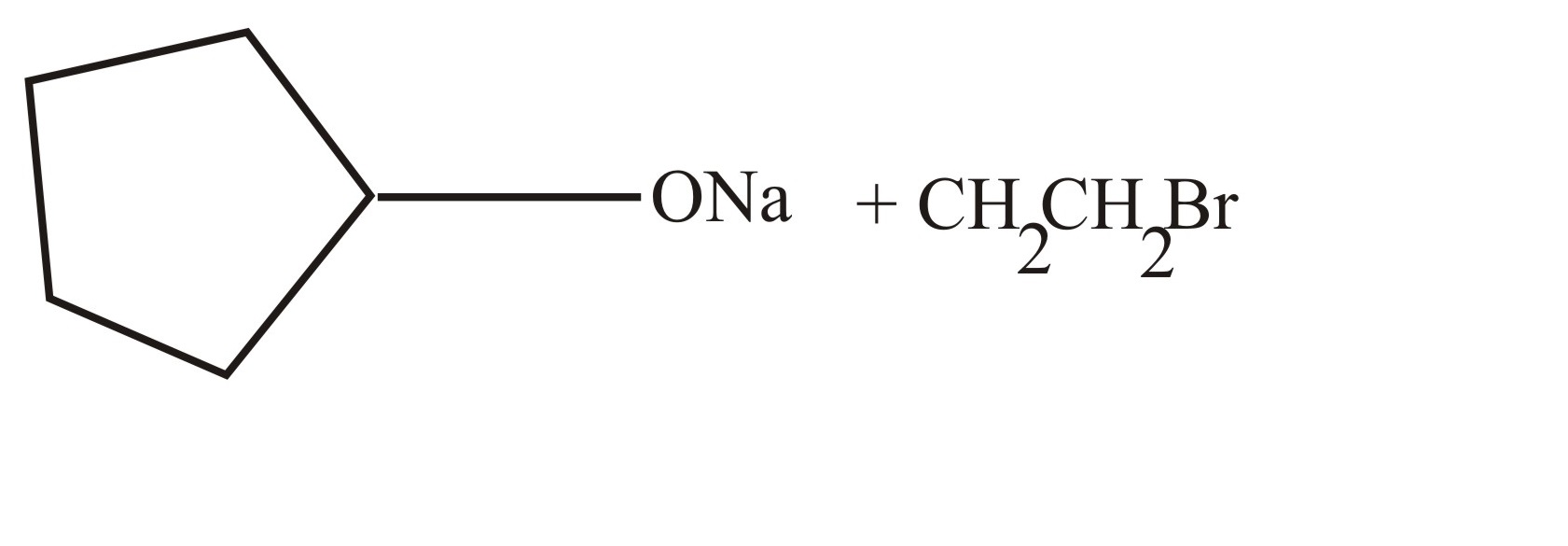
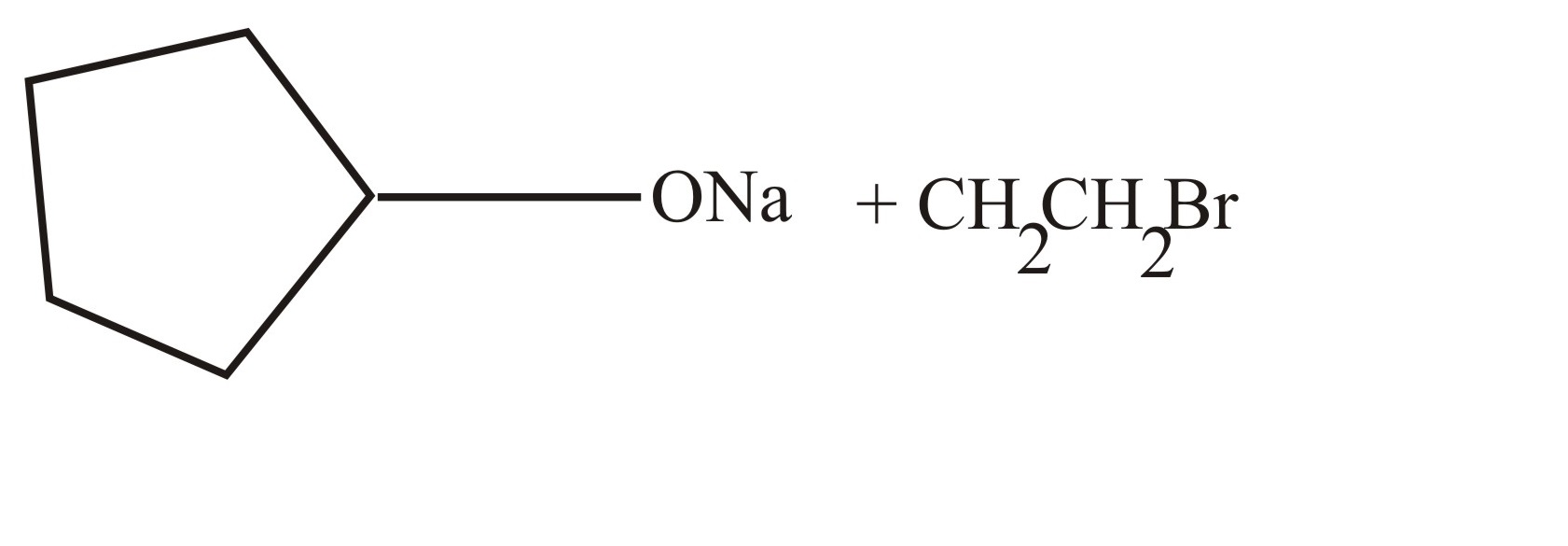
(b)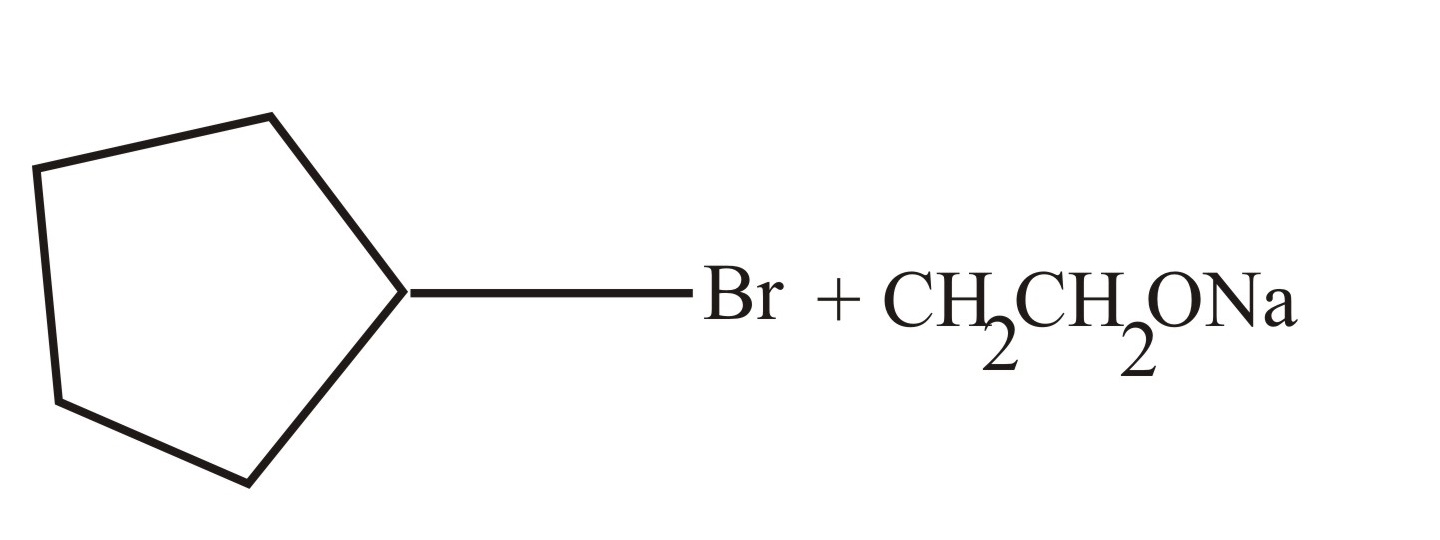
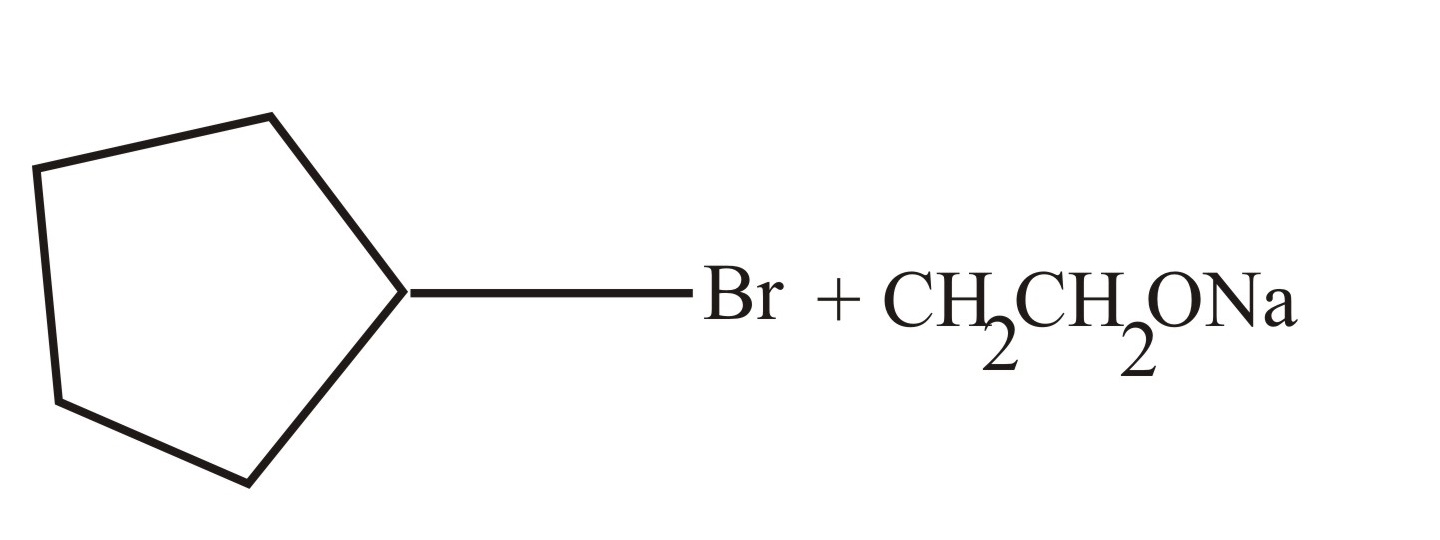
(c)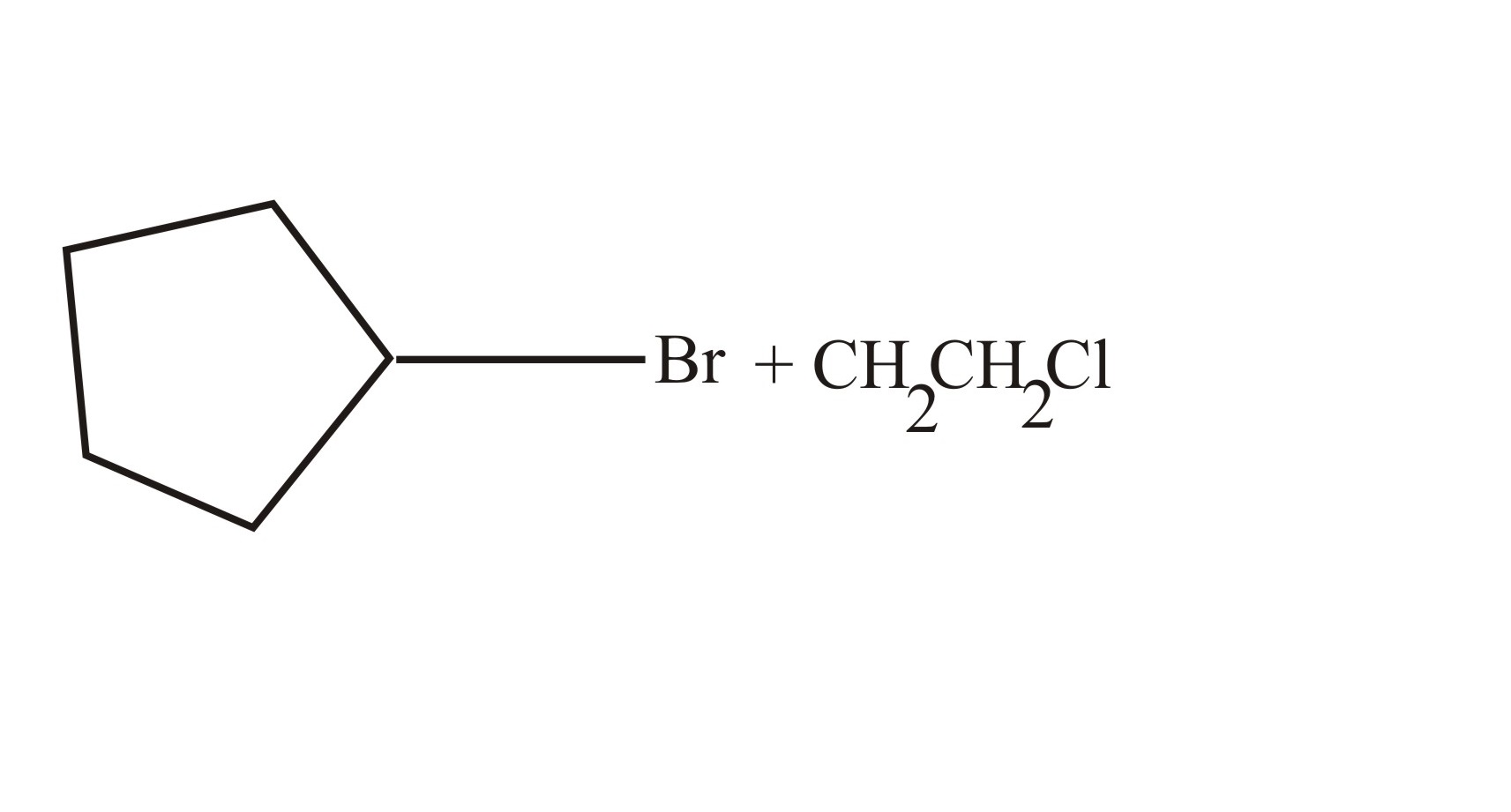

(d)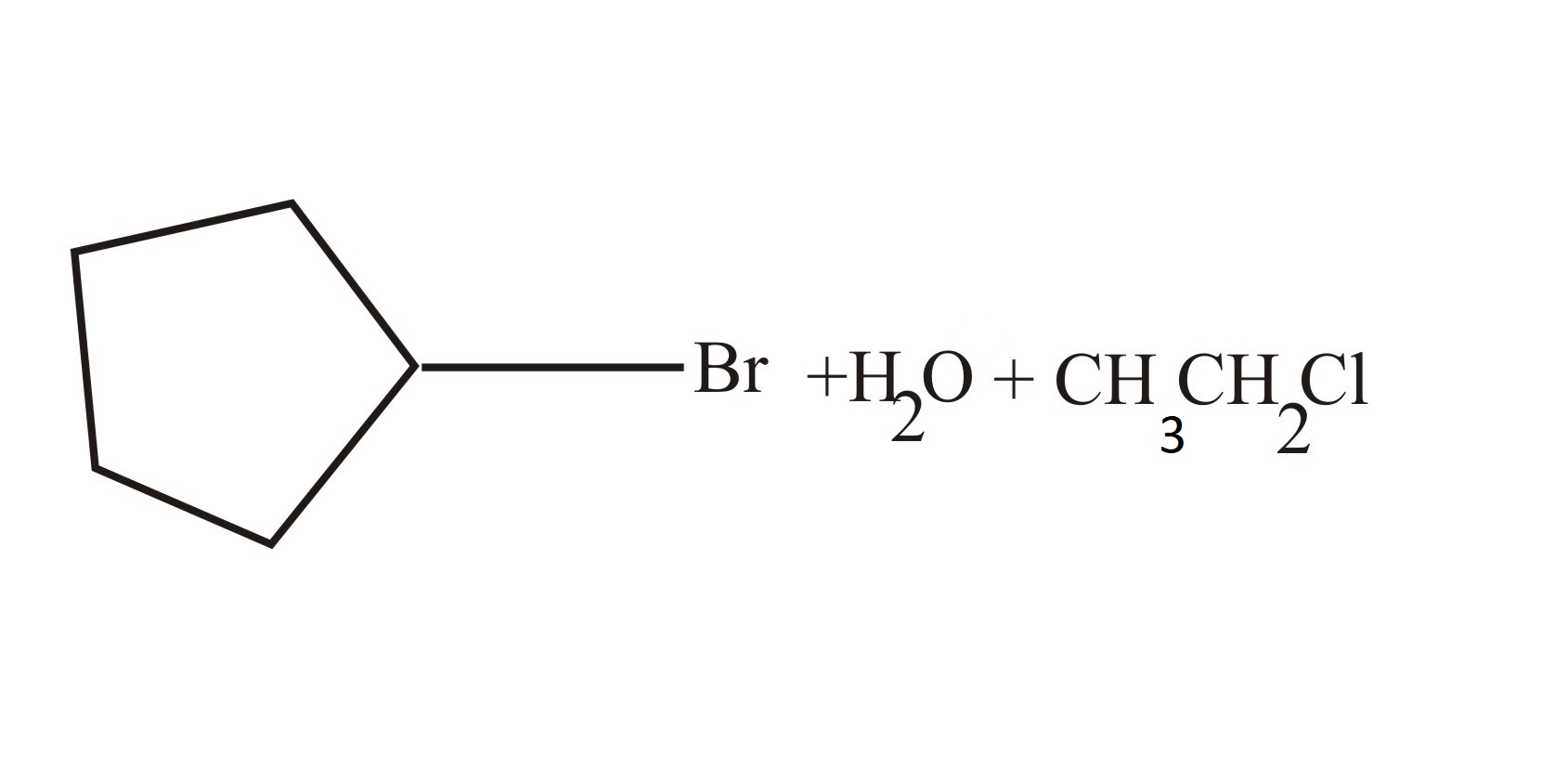
50% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
MEDIUM
The major product of the following reaction is
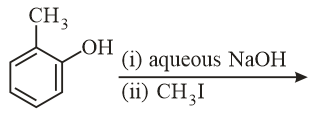
EASY
EASY
HARD
MEDIUM
MEDIUM
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Synthesis of ethyl phenyl ether may be achieved by Williamson synthesis.
Reason (R): Reaction of bromobenzene with sodium ethoxide yields ethyl phenyl ether.
In the light of the above statements, choose the most appropriate answer from the options given below.
EASY
MEDIUM
MEDIUM
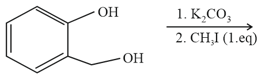
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
The following compound
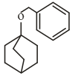
can readily be prepared by Williamson ether synthesis by reaction between
MEDIUM
EASY
MEDIUM
MEDIUM
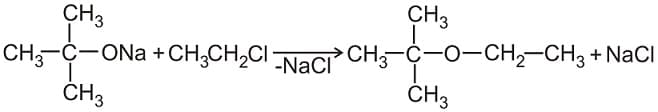
is called:
MEDIUM
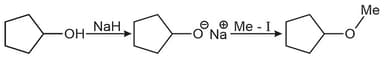
Can be classified as:

