EASY
Earn 100
Which of the following molecules gives 'Glyoxal' as one of the product on reductive ozonolysis?
(a)
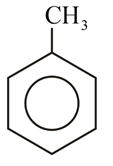
(b)
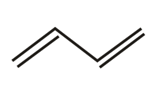
(c)
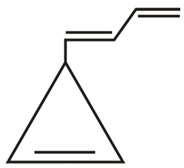
(d)
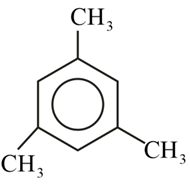
50% studentsanswered this correctly
Important Questions on Unsaturated Hydrocarbons- Alkenes
HARD
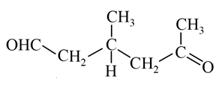
is obtainable from ozonolysis of which of the following cyclic compounds?
MEDIUM
The product of which of the following reactions forms a reddish brown precipitate when subjected to Fehling's test?
MEDIUM
EASY
HARD
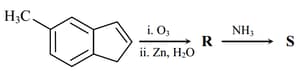
EASY
MEDIUM
MEDIUM
HARD
Compound P and R upon ozonolysis produce Q and S, respectively. The molecular formula of Q and S is . Q undergoes Cannizzaro reaction but not haloform reaction, whereas S undergoes haloform reaction but not Cannizzaro reaction.
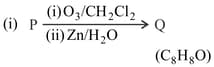
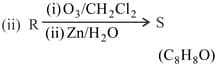
MEDIUM
(3-oxo-hexanedicarboxylic acid) will be:
MEDIUM
EASY
HARD
Identify the major product in the following reaction
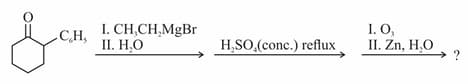
HARD
HARD
MEDIUM
MEDIUM
HARD
In the following reaction-
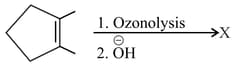
The major product is-
HARD