Which of the following organic compound is least soluble in water.

Important Points to Remember in Chapter -1 - Aldehydes, Ketones and Carboxylic Acids from Embibe Experts Achieve CUET (UG) Chemistry Practice Book Solutions
(i) By oxidation of alcohols :
(ii) By dehydrogenation of alcohols :
(iii) By ozonolysis of alkenes :
(iv) By hydration of alkynes :
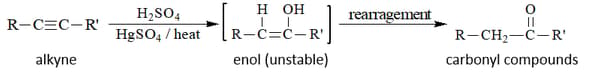
2. Preparation of Aldehydes
(i) Rosenmund reduction :
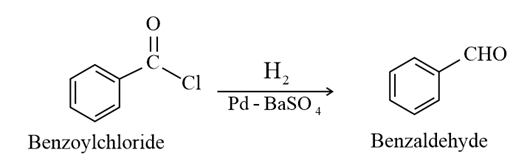
(ii) Stephen reaction:
(iii) Nitriles are selectively reduced by DIBAL-H and followed by hydrolysis gives aldehydes
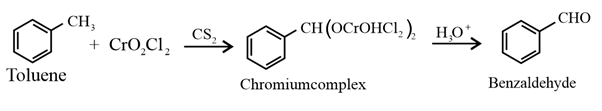
(iv) Aromatic aldehydes (benzaldehyde and its derivatives) are prepared from aromatic hydrocarbons by Etard reaction:
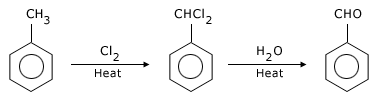
(vi) Aromatic aldehydes also prepared by side chain chlorination of alkyl benzene followed by hydrolysis.
(vii) Gatterman – Koch reaction :
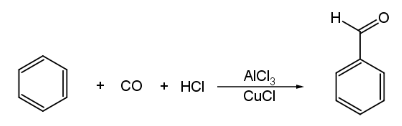
3. Preparation of Ketones
(i) Treatment of acyl chlorides with dialkylcadmium and reaction of cadmium chloride with Grignard reagent gives ketones.
(ii) Treating a nitrile with Grignard reagent followed by hydrolysis yields a ketone.
(iii) Friedel-Crafts acylation reaction:
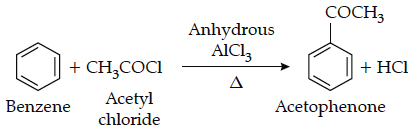
4. Chemical reactions of aldehydes and ketones:
(i) Aldehydes and ketones undergo nucleophilic addition reactions onto the carbonyl group with a number of nucleophiles such as, alcohols (or diols), ammonia derivatives, and Grignard reagents.
(ii) The -hydrogens in aldehydes and ketones are acidic. Hence, aldehydes and ketones having at least one -hydrogen, undergo Aldol condensation in the presence of a base to give -hydroxyaldehydes (aldol) and -hydroxyketones(ketol), respectively.
(iii) Aldehydes having no -hydrogen undergo self oxidation and reduction (disproportionation) reaction on heating with concentrated alkali. This is cannizzaro reaction.
(iv) Aldehydes and ketones are reduced to alcohols with , or by catalytic hydrogenation.
(v) The carbonyl group of aldehydes and ketones can be reduced to a methylene group by Clemmensen reduction or Wolff-Kishner reduction.
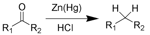
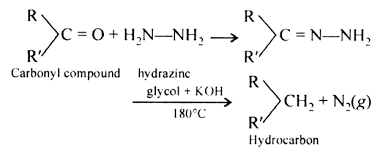
(vi) Distinguishing aldehydes from ketones:
(a) Tollen’s test
(b) Fehling’s test
5. Preparation of Carboxylic Acids
(i) From primary alcohols and aldehydes
(ii) Aromatic carboxylic acids can be prepared by vigorous oxidation of alkyl benzenes with chromic acid or acidic or alkaline potassium permanganate.
(iii) Nitriles are hydrolysed to amides and then to acids in the presence of or as catalyst.
(iv) From Grignard reagents :
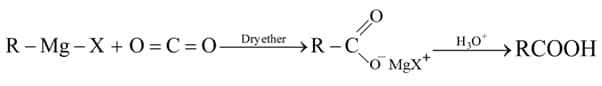
6. Chemical properties of carboxylic acids:
(i) Carboxylic acids are considerably more acidic than alcohols and most of simple phenols.
(ii) Carboxylic acids are reduced to primary alcohols with or better with diborane in ether solution and also undergo α-halogenation with and in the presence of red phosphorus (Hell-Volhard Zelinsky reaction).
(iii) Carboxylic acids lose carbon dioxide and produce hydrocarbons on reacting with soda lime. This reaction is called decarboxylation reaction.
(iv) Aromatic carboxylic acids undergo electrophilic substitution reactions and give meta substituted products as group acts as meta directing group.
